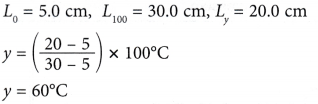Which Instrument is Used to Measure the Temperature?
- Temperature is a measure of the degree of hotness of an object.
- A hot object has a higher temperature than a cold object.
- When two objects are in thermal contact, heat energy is transferred from the object at the higher temperature to the object at the lower temperature.
- Table shows a comparison between temperature and heat.
Temperature Heat Degree of hotness of an object Energy transferred from a hot object to a cold object. A base quantity A derived quantity SI unit is kelvin (K) SI unit is joule (J) A scalar quantity A scalar quantity - Temperature is measured by a thermometer. The most commonly used thermometer is the liquid- in-glass thermometer.
- A liquid-in-glass thermometer consists of a capillary tube with a thin-walled bulb at one end. It usually contains a fixed mass of mercury or alcohol.
- When the temperature increases, the volume of the mercury increases.
- This causes the mercury to expand into the capillary tube. Therefore, the length of the mercury column increases as temperature increases.
- The change in the length of the mercury column is proportional to the change in the temperature.
- The laboratory liquid-in-glass thermometers use the Celsius scale.
- The Celsius scale is defined by two fixed points of temperature – the ice point and the steam point. Table gives the definition and values of the two fixed points.
Fixed point Definition Value Lower fixed point: Ice point The temperature of pure melting ice 0°C Upper fixed point: Steam point The temperature of steam from water that is boiling under standard atmospheric pressure 100°C - The liquid-in-glass thermometer uses the concept of thermal equilibrium to measure temperature.
(a) When a thermometer is placed into an object, energy is transferred between the thermometer and the object until thermal equilibrium occurs.
(b) The temperature of the thermometer is now equal to the temperature of the object.
(c) Therefore the reading of the thermometer is the temperature of the object.
Calibration of Thermometers
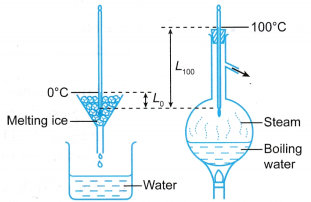
A new thermometer without a scale can be calibrated by placing the bulb in ice and then in steam, as shown in Figure.
 Step 1
Step 1
- Place the bulb into melting ice.
- Wait until the mercury column stops moving.
- There is now thermal equilibrium at 0°C.
- Mark 0°C on the stem.
Step 2
- Place the bulb into steam.
- Wait until the mercury column stops moving.
- There is now thermal equilibrium at 100°C
- Mark 100°C on the stem.
Step 3
- Divide the region between the 0°C mark and the 100°C mark into 100 equal divisions.
- Label the scale of the thermometer from 0, 10, 20, …, to 100.
- The thermometer is calibrated and ready to measure temperature.
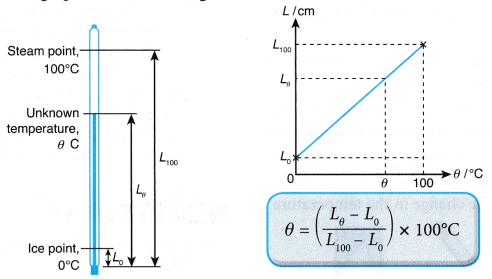
- If the scale of the thermometer is not marked, the temperature of an object can be determined from the formula or calibration graph, as shown in Figure.
- Lower temperatures can be measured by liquid- in-glass thermometers filled with alcohol, which freezes at -115°C and boils at 78°C. The alcohol used needs to be coloured.
- For extra low temperature up to -200°C, liquid pentane is used instead of alcohol.
- Water is not suitable for use in thermometers because
(a) it freezes at 0°C,
(b) it does not expand regularly. - The sensitivity of a thermometer can be increased by:
(a) Decreasing the diameter of the capillary tube in the stem. This will cause the mercury column to move through a longer distance in the tube as it expands.
(b) Using a bulb with thinner walls. Heat energy can be transferred at a faster rate into the mercury. The thermometer will respond faster to changes in temperature.
Calibration of Thermometers Example Problems with Solutions
Example 1. Figure shows a thermometer being used to measure the temperature of some sand.
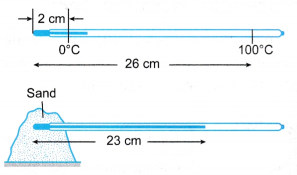 What is the temperature of the sand?
What is the temperature of the sand?
Solution:
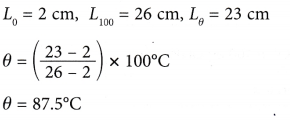
Example 2. Figure shows the calibration graph of a mercury thermometer.
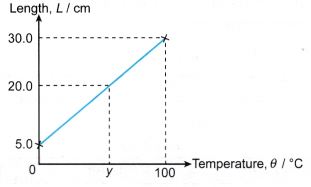
What is the temperature, y ?
Solution: