How many periods are in the periodic table?
- Each horizontal row of elements in the Periodic Table is known as a period.
- The Periodic Table consists of seven periods, from Period 1 to Period 7.
- Table shows the changes in the proton numbers and number of valence electrons when going across Period 2.
Element in Period 2 Li Be B C N O F Ne Proton number 3 4 5 6 7 8 9 10 Electron arrangement
2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 Number of valence electrons 1 2 3 4 5 6 7 8 - When going across Period 2 and Period 3 from left to right
- the proton number increases by one from one element to the next
- the number of valence electrons increases by one from one element to the next.
- When going across Period 2 and Period 3 both the physical and chemical properties vary in a certain pattern.
- The trend of changes in the properties of the elements across a period can be used to predict the properties of an element in Period 2 and Period 3.
Change in properties across Period 3
Table shows some properties of the elements in Period 3.
| Element | Na | Mg | Al | Si | P | S | Cl | Ar |
| Proton Number | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| Electron arrangement | 2.8.1 | 2.8.2 | 2.8.3 | 2.8.4 | 2.8.5 | 2.8.6 | 2.8.7 | 2.8.8 |
| Atomic radius (pm) | 186 | 160 | 143 | 118 | 110 | 104 | 100 | 94 |
| Electronegativity | 0.9 | 1.2 | 1.5 | 1.8 | 2.1 | 2.5 | 3.0 | – |
| Melting point (°C) | 98 | 649 | 660 | 1411 | 44 | 113 | -101 | -189 |
| Boiling point (°C) | 886 | 1090 | 2467 | 2355 | 280 | 444 | -35 | -186 |
Atomic radius (atomic size)
- The atomic radius (atomic size) decreases when going across a period from left to right.
- This can be explained by using Period 3 as an example, as shown below.
- All atoms of the elements in Period 3 have 3 shells occupied with electrons, as shown by the electron arrangements in above Table.
- The proton number increases across Period 3 from left to right. This causes an increase in the positive charge of the nucleus when going across Period 3, as shown in Table.
Element Na Mg Al Si P S Cl Ar Charge of nucleus +11 +12 +13 + 14 +15 + 16 +17 +18 - The pulling force exerted by the increasing nuclear charge on the electrons in the first three occupied shells becomes stronger and these electrons are pulled closer to the nucleus when going across Period 3.
- As a result, the atomic radius (atomic size) decreases when going across Period 3.
Electronegativity
- The electronegativity of an element is a measurement of the strength of an atom of that element in a molecule to attract electrons towards its nucleus.
- The electronegativity of elements increases when going across a period from left to right.
- This can be explained as follows:
- The positive charge of the nucleus increases across a period (Period 2 or Period 3) due to an increase in the proton numbers.
- The atomic radius decreases across a period (Period 2 or Period 3).
- The increase in positive nuclear charge and the decrease in atomic size across a period cause an increase in the strength of the nucleus to attract electrons. Therefore, the electronegativity increases.
- In Period 2 or 3, metals on the left are less electronegative, whereas non-metals on the right are more electronegative.
Physical states
- Elements in Period 2 and Period 3 change from solid to gas when going across the period.
- Metals on the left are solids because their melting and boiling points are high.
- (i) Non-metals on the right are usually gases because their melting and boiling points are low.
(ii) However, some non-metals such as carbon from Period 2 as well as silicon, phosphorus and sulphur from Period 3 are solids. - Table shows the physical states of the elements in Period 2 and Period 3.

Metallic properties
- The metallic properties of the elements decrease when going across Period 2 or Period 3.
- The metallic properties of an element is measured by its electropositivity.
- In a period, metals on the left are more electropositive whereas non-metals on the right are less electropositive.
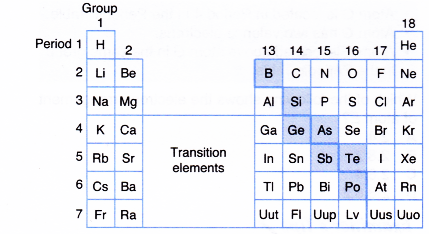
- The shaded elements in Figure have both metallic and non-metallic properties.
- These elements are known as metalloids or semimetals.
- For example, boron, silicon, germanium, arsenic, antimony, tellurium and polonium are semimetals or metalloids.
Semimetals (metalloids) means elements intermediate in properties between metals and non-metals. - Elements on the left of the Periodic Table are metals and elements on the right of the Period Table are non-metals.
- When going across a period, the metallic properties on the left change to semi-metallic properties and finally to non-metallic properties on the right.
Electrical conductivity
Table shows the electrical conductivities of the elements when going across Period 3.
| Na Mg Al | Good conductors of electricity |
| Si | A weak conductor of electricity but becomes a good conductor of electricity (semiconductor) when doped with a small amount of boron or phosphorus. |
| P S Cl Ar | Cannot conduct electricity |
Based on the above electrical conductivities, the elements in Period 3 can be classified into metals, semimetal (or metalloid) and non-metals as shown in Figure.

Properties of the oxides of elements in Period 3
- The elements in Period 3 can be classified into metals or non-metals based on the basic or acidic properties of their oxides.
- The following ways are used to classify the Period 3 elements.
- Metals form oxides with basic properties only.
- Some metals can form oxides with both acidic and basic properties. These oxides are known as amphoteric oxides.
- Non-metals form oxides with acidic properties only.
- The properties of the oxides of elements in Period 3 change from basic to amphoteric properties and then acidic properties when going across the period.
Investigating the properties of period 3 oxides experiment
Aim: To investigate the properties of the oxides of elements in Period 3.
Problem statement: How do the properties of the oxides of elements in Period 3 change across the period?
Hypothesis: The acidic properties of the oxides of elements increase, whereas basic properties of the oxides of elements decrease when going across Period 3.
Variables:
(a) Manipulated variable : Types of oxides of elements in Period 3
(b) Responding variables: pH values in water, solubility of the oxides in acid and alkali
(c) Controlled variable : Water, nitric acid, sodium hydroxide solution
Operational definition:
- Oxides that dissolve in water to form solutions with pH values less than 7 are acidic and pH values more than 7 are alkaline.
- Oxides that dissolve in an acid exhibit basic properties.
- Oxides that dissolve in an alkali exhibit acidic properties.
- Oxides that dissolve in both acid and alkali exhibit amphoteric properties.
Materials: Sodium oxide, Na2O, magnesium oxide, MgO, aluminium oxide, Al2O3, silicon(IV) oxide, SiO2, phosphorus pentoxide, P4O10, sulphur dioxide, SO2 gas in a covered gas jar, dichlorine heptoxide, Cl2O7, Universal Indicator, 2 mol dm-3 nitric acid, 2 mol dm-3 sodium hydroxide solution and distilled water.
Apparatus: Boiling tubes, test tubes, 100 cm3 measuring cylinder, Bunsen burner, test tube holder, glass rod and
spatula.
Procedure:
A. Acidic/basic properties of the oxides of elements in Period 3
- A small amount of sodium oxide powder is added to 2 cm3 of distilled water in a test tube. The mixture is stirred well with a glass rod until no further change occurs.
- Two drops of Universal Indicator are then added and shaken well. The pH of the solution is then recorded.
- Steps 1 to 2 are repeated using Steps 1 to 2 are repeated using MgO, Al2O3, SiO2, P4O10, SO2 and Cl2O7 to replace sodium oxide. For sulphur dioxide gas, the gas is bubbled through 2 cm3 of distilled water in a test tube.
B. Amphoteric properties of the oxides of elements in Period 3
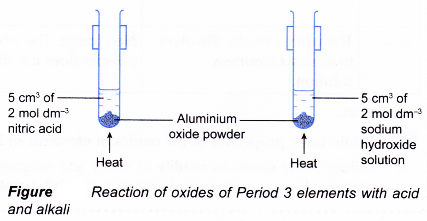
- 5 cm3 of 2 mol dm-3 nitric acid and 5 cm3 of 2 mol dm-3 sodium hydroxide solution are placed in two separate boiling tubes respectively.
- A small amount of aluminium oxide powder is added to each of the two boiling tubes.
- The boiling tubes are warmed and shaken well until no further change occurs.
- All the changes are recorded.
- Steps 1 to 4 are repeated using magnesium oxide and silicon(IV) oxide respectively to replace aluminium oxide.
Results:
A. Acidic/basic properties of the oxides of elements in Period 3
| Oxide | Observation | Inference | |
| With water | With Universal Indicator | ||
| Na20 | The white powder dissolves in water to form a colourless solution. | pH of the solution formed = 13 to 14 | The solution formed is a strong alkali. Sodium oxide is a basic oxide. Hence, sodium exhibits metallic properties. |
| MgO | The white powder dissolves slightly to form a colourless solution. Most of the white powder does not dissolve in water. | pH of the solution formed = 8 to 9 | The solution formed is a weak alkali. Magnesium oxide is a basic oxide. Hence, magnesium exhibits metallic properties. |
| Al2O3 | The white powder does not dissolve in water. | No change (pH = 7) | – |
| SiO2 | The white powder does not dissolve in water. | No change (pH = 7) | – |
P4O10 | The white powder dissolves to form a colourless solution. | pH of the solution formed = 2 to 3 | The solution formed is acidic. Phosphorus pentoxide is an acidic oxide. Hence, phosphorus exhibits non-metallic properties. |
| SO2 | The colourless gas dissolves to form a colourless solution. | pH of the solution formed = 3 | The solution formed is acidic. Sulphur dioxide is an acidic oxide. Hence, sulphur exhibits non- metallic properties. |
| Cl2O7 | The liquid dissolves to form a colourless solution. | pH of the solution formed = 1 | The solution formed is a strong acid. Dichlorine heptoxide is an acidic oxide. Hence, chlorine exhibits non- metallic properties. |
B. Amphoteric properties of the oxides of elements in Period 3
| Oxide | Observation | Inference | |
| With sodium hydroxide solution | With dilute nitric acid | ||
| Al2O3 | The white powder dissolves to form a colourless solution. | The white powder dissolves to form a colourless solution. | Aluminium oxide shows acidic and basic properties, that is amphoteric properties. Hence, aluminium as a metal, forms an amphoteric oxide. |
| MgO | No change. The white powder does not dissolve. | The white powder dissolves to form a colourless solution. | Magnesium oxide shows basic properties only. Hence, magnesium as a metal, forms a basic oxide. |
| SiO2, | The white powder dissolves to form a colourless solution. | No change. The white powder does not dissolve. | Silicon(IV) oxide shows acidic properties only. Hence, silicon as a non-metal, forms an acidic oxide. |
Discussion:
A. Acidic/basic properties of the oxides of elements in Period 3
- Sodium oxide dissolves readily in water and magnesium oxide dissolves slightly in water to form alkaline solutions. Hence, sodium and magnesium exhibit metallic properties.
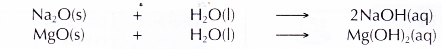
- Aluminium oxide and silicon(IV) oxide are insoluble in water.
- Phosphorus pentoxide, sulphur dioxide and dichlorine heptoxide dissolve in water to form acidic solutions. Hence, phosphorus, sulphur and chlorine exhibit non-metallic properties.

B. Amphoteric properties of the oxides of elements in Period 3
- All acidic oxides can dissolve in alkalis to form salt and water, whereas all basic oxides can dissolve in acids to form salt and water. These reactions are known as neutralisation.
- Magnesium oxide can only dissolve in dilute nitric acid but cannot dissolve in sodium hydroxide solution. Hence, magnesium oxide exhibits basic properties only.

- Aluminium oxide can dissolve in both dilute nitric acid and sodium hydroxide solution. Hence, aluminium oxide exhibits both basic and acidic properties, that is, amphoteric properties.

- Silicon(IV) oxide cannot dissolve in dilute nitric acid but can dissolve in sodium hydroxide solution. Hence, silicon(IV) oxide exhibits acidic properties only.

- The properties of the oxides of elements in Period 3 can be summarised as shown in Table.
Oxides of elements in Period 3 Na2O MgO Al2O3 SiO2 P4O10 SO2 Cl2O7 Properties of oxides Basic Basic Amphoteric Acidic Acidic Acidic Acidic
Conclusion:
When going across Period 3 from left to right, the properties of the oxides of elements change from basic to acidic. Hence, the hypothesis can be accepted.
Uses of semimetals (metalloids) in industry
- Silicon, as a semimetal (metalloid), is a weak conductor of electricity.
- In industry, silicon is doped with boron or phosphorus so that it becomes a good conductor of electricity known as semiconductor.
- Semiconductors are used in the microelectronic industry to make diodes, transistors, and other electronic components.
- These electronic components are used to make microchips which are integrated circuits containing many electronic components on small thin pieces of silicon wafer.
- Microchips are widely used in the manufacture of computers, calculators, cell phones, video cameras, video recorders, televisions and other microelectronic equipments.
- Apart from silicon, germanium is another semimetal or metalloid used in the microelectronic industry.