How polymers are classified?
Polymers
- Polymers are long chained molecules formed by joining up many identical repeating sub-units called monomers.
- Polymerisation is a process by which the monomers are joining together into chain-like big molecules known as polymers.
- Polymerisation can be represented graphically as shown below.

where M represents the monomer and n is a very big integer - A polymer is a macromolecule and may consist of thousands of monomers. Hence it normally has a large relative molecular mass.
- The properties of a polymer are very different from the properties of its monomer.
- Polymers can be divided into two types: natural polymers and synthetic polymers.
- Examples of natural polymers: DNA, proteins, cellulose, starch and natural rubber.
Examples of synthetic polymers: poly(ethene), polystyrene, nylon and polyesters
People also ask
- Justify the uses of synthetic polymers in daily life
- What are Synthetic Fibres and give some Examples
- What are the Advantages and Disadvantages of Synthetic Fibres
- Is Polyester Synthetic or Artificial
- What is the most common type of plastic?
- How is glass produced from raw materials?
- How ceramics are made?
- How is a composite material made?
Naturally occurring polymers
- Naturally occurring polymers are polymers obtained from living things such as plants and animals.
- Examples of naturally occurring polymers are: natural rubber, cellulose, starch and silk.
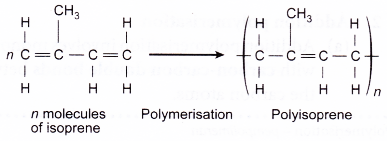
- Natural rubber is obtained from rubber trees, Hevea brasiliensis as latex. The monomer for natural rubber is isoprene or 2-methyl-but-1,3- diene. Each isoprene unit has two double bonds and undergoes addition polymerisation to form polyisoprene or natural rubber.
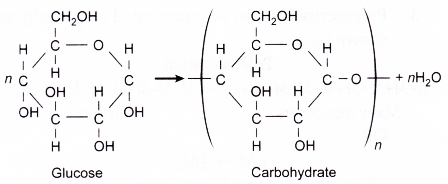
- Carbohydrates such as starch and cellulose are formed by plants through polymerisation from a simple sugar called glucose.
- Wool, meat and silk are made up of a variety of proteins. They are all naturally occurring polymers that consist of amino acid as the monomer.
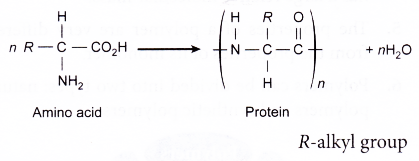
Proteins:
- The building blocks (monomers) of proteins are the amino acids.
- The general structural formula of an amino acid is given below.
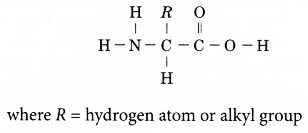
- Amino acids are linked together by a condensation polymerisation to form various protein polymers. The condensation reaction joins together the carboxyl group and amino group and a molecule of water is eliminated. A peptide linkage is formed.
Carbohydrates:
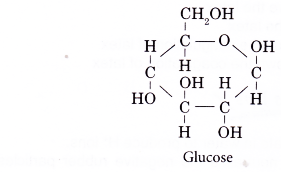
- Glucose molecule is the monomer of carbohydrates.
- The molecular formula of glucose is C6H12O6 and its structure is shown below.
- A condensation reaction joins thousands of glucose molecules to form two types of complex carbohydrate polymers:
(a) Starch
(b) Cellulose - Two hydroxyl groups from adjacent glucose monomers link up with the elimination of a water molecule.
- The following shows the structure of starch.
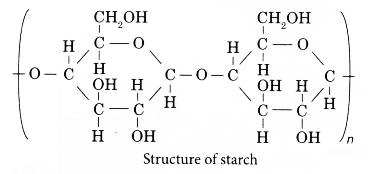
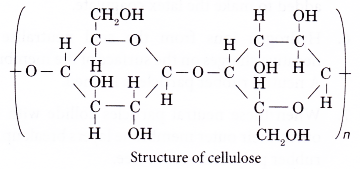
- The structure of cellulose is as shown below.
Synthetic polymers
- Through continuous research and development, scientists are able to copy the structure of naturally occurring polymers to produce synthetic polymers.
- Synthetic polymers are polymers made by man through chemical reactions in industry from polymerisation of monomers.
- The monomers are usually obtained from the fractional distillation of petroleum after going through refining and cracking processes.
- Synthetic polymers are commonly known as plastics.
- Examples of synthetic polymers are: polyethene, polypropene, polyvinyl chloride or PVC, polystyrene, perspex, nylon and terylene.
How are synthetic polymers made?
- Synthetic polymers are made through two types of polymerisation:
(a) Addition polymerisation
(b) Condensation polymerisation - Addition polymerisation
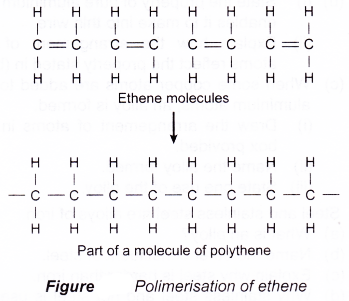
(a) Addition polymerisation involves monomers with carbon-carbon double bonds between the carbon atoms.
(b) During addition polimerisation, the double bonds between pairs of carbon atoms break and the carbon atoms of adjacent molecules join together.
(c) Examples of monomer that can undergo
addition polymerisation are: ethene, chloroethene and styrene. They undergo addition polymerisation to form polyethene, polychloroethene and polystyrene respectively.
(d) Polymerisation of ethene is shown in Figure. - Condensation polymerisation
(a) Condensation polymerisation involves the joining up of monomers with the formation of other smaller and simple molecules such as water.
(b) Examples of polymers produced by condensation are nylon and terylene. - Synthetic polymers are use to make plastics, synthetic fibres and synthetic rubbers.