What was Rutherford’s Original Hypothesis
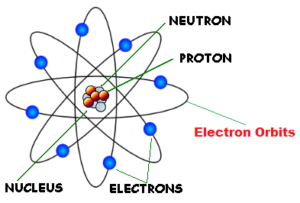
Rutherford model of an atom
Rutherford and his co-workers made a fundamental contribution in understanding the structure of the atom and establishing the presence of a small nucleus in the atom. They performed a number of experiments known as scattering experiments. They took very thin sheets of gold foil (only 4 × 10–5 cm thick) and bombarded it with a stream of alpha (a) particles. The alpha particles are positively charged helium ions (He2+) which carry two units of positive charge and mass four times that of an atom of hydrogen (i.e., mass of helium ions = 4 a.m.u.). These are emitted from radioactive elements such as radium.
Following important observations :
- Most of the fast moving a-particles passed straight through the gold foil without any deflection from their original path.
- Some of the a-particles were deflected from their path through small angles.
- Very few (about 1 in 12,000) did not pass through the foil at all but suffered large deflections (more than 90º) or even came back in the direction from which they came.
People also ask
- How would you describe the Structure of an Atom
- What did Bohr Contribute to the Theory of an Atom
- What are the Characteristics of Electron, Proton and Neutron
- Explain Bohr Bury rules for Distribution of Electrons into Different Shells
- What are the Isotopes, Isobars and Isotones of an Element
- What did Dalton Contribute to the Understanding of the Atom
- What is the Definition of Atom and Molecule
- What is Atomic Mass
- How has the Model of the Atom Changed Over the Years?
The main conclusions of Rutherford’s experiment :
Most of the space inside the atom is empty. Therefore, most of the a-particles went through the gold foil without deflecting from their path.
There is a positive tiny part in the atom in its centre, which deflects or repels the a-particles. This must be containing the whole mass of the atom. Moreover, this mass must be occupying a very small space within the atom because only a few a-particles suffered large deflections. This positively charged heavy mass in the centre of the atom is called nucleus. The a-particle get deflected from their normal path when they came close to nucleus due to force of repulsion (similar charges).
The deflections of the a-particles to large angles indicate that the a-particles has direct collision with the positively charged nucleus.
It was observed that the volume of the nucleus is very small as compared to the total volume of the atom. The radius of the atom is of the order of 10–10 m, while the radius of the nucleus has been estimated to be of the order of 10–15 m. This means that the size of the nucleus is extermely small i.e., about 105 times less than the size of the atom.
Rutherford’s Atomic model : The main features of Rutherford’s model of an atom are :
- The atom consists of a positively charged centre called the nucleus.
- Most of the mass of the atom is concentrated in the nucleus.
- The volume of the nucleus is very small as compared to the total volume of the atom.
- The nucleus is surrounded by the negatively charged electrons. the electrons balance the positive charge of the nucleus. Therefore, the number of electrons in an atom is equal to the number of protons in it.
- The magnitude of the positive charge on the nucleus is different for different atoms.
- The electrons are not stationary but they are revolving around the nucleus at very high speeds like planets revolving around the sun. As a result, the electrons are also known as planetary electrons.
Drawbacks of Rutherford’s model :
Rutherford model failed in view of electromagnetic theory given by Maxwell. According to this theory a charged particle when accelerated emits energy in the form of electromagnetic radiation. According to Rutherford’s model, electrons are revolving around the nucleus. This means, electrons would be in a state of acceleration all the time. Since electrons are charged particles, therefore, electron revolving in an orbit should continuously emit radiations. As a result of this, it would slow down and would no longer be able to withstand the attractive force of the nucleus. Hence, it would move closer and closer to the nucleus and would finally fall in the nucleus by following a spiral path. This means atom would collapse. But actually we know atom is stable. Thus, Rutherford’s model failed to explain stability of atoms.