Kerala Plus One Chemistry Previous Year Question Paper March 2017 with Answers
| Board | SCERT |
| Class | Plus One |
| Subject | Chemistry |
| Category | Plus One Previous Year Question Papers |
Time Allowed: 2 hours
Cool off time: 15 Minutes
Maximum Marks: 60
General Instructions to Candidates
- There is a ‘cool off time’ of 15 minutes in addition to the writing time.
- Use the ‘cool off time’ to get familiar with questions and to plan your answers.
- Read the instructions carefully.
- Read questions carefully before answering.
- Calculations, figures and graphs should be shown in the answer sheet itself.
- Malayalam version of the questions is also provied.
- Give equations wherever necessary.
- Electronic devices except non programmable calculators are not allowed in the Examination Hall.
Question 1:
a. Determine the number of moles present in 0.55 mg of electrons.
i. 1 mole
ii. 2 moles
iii. 1.5 moles
iv. 0.5 mole [1]
b. Give the empirical formula of the following. [2]
C6H12O6, C6H6. CH3COOH, C6H6C16
c. Two elements, carbon and hydrogen combine to form C2H6, C2H4 and C2H2. Identify the law illustrated here. [1]
Question 2:
a. i. Write the electronic configuration of chromium (Z=24).
ii. Find the number of electrons in the subshells with azimuthal quantam number l=2.
iii. Represent the orbital with quantum numbers n = 1 and 1 = 0.
b. Give the mathematical representation of Heisenberg’s uncertainty principle and its one important significance.
Question 3:
Electron gain enthalpy is one of the important periodic properties.
a. Define electron gain enthalpy. [1]
b. Explain any two factors affecting electron gain enthalpy. [2]
c. Write the oxidation state and covalency of Al in (A/ F6)3- [1]
Question 4:
The geometry of the molecule is decided by type of hybridization.
a. Discuss the shape of PCl5 molecule using hybridization. [2]
b. Give the reason for the high reactivity of PCl5 [2]
c. Isoelectronic species have the samebond order. Among the following, choose the pair having same bond order. Among the following, choose the pair having same bond order.
CN–, O2– ,NO+, CN+
Question 5:
a. Give the reason behind the following.
i. The glass window pannels of old buildings are thicker at the bottom than at the top. [1]
ii. Sharp glass edges are heated for making them smooth. [1]
b. Maxwell and Boltzmann have shown that actual distribution of molecular speeds depends, on temperature and molecular mass.
i. What do you mean by most probable velocity? [1]
ii. At the same temperature which will move faster, N2 or Cl2? [1]
Question 6:
a. Some macroscopic properties are given below. Help Reena to classify them into two groups under suitable titles.
[Heat capacity, Entropy, Refractive in¬dex, Surface tension.] [2]
b. For the reaction
2A(g) + 2B(g) → 2D(g)
∆U°= -10.5 kJ/mol
∆S°= – 44.1J/k/mol at 298 K.
Calculate ∆G° for the reaction. [2]
Question 7:
a. Classify the following solutions into acidic, basic and neutral
NaCl, NH4NO3, NaCN, NaNO2 [2]
b. pH of blood remains constant in spite of the variety of goods and spices we eat. Give a reason. [1]
c. The solubility of Mg(OH)2 at 298K is 1.5 x 10-4. Calculate the solubility product.[2]
Question 8:
Permanganate ion reacts with bromide ion in basic medium to give manganese dioxide and bromate ion. Write the balanced equa¬tion for the reaction using the oxidation number method.
Skeletal equation is
MnO4– + Br– → MnO2 + BrO3– [3]
Question 9:
Hydrogen is the most abundant element in the universe, But in free state it is almost not found in earth’s atmosphere.
a. Suggest any three methods for the preparation of H2 gas by selecting suitable substance given below.
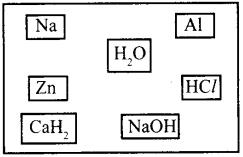
b. Do you expect carbohydrides of the type CnH2n+2to act as Lewis acid or base? Why?
Question 10:
The s-block of the periodic table constitutes alkali metals and alkaline earth metals.
a. The hydroxides and carbonates of sodium and pottassium are more soluble than that of corresponding salts of Magnesium and Calcium. Explain. [2]
b. Write the chemical name of the following:
i. Caustic soda
ii. Baking soda
iii. Slaked lime
iv. Milk of lime [2]
Question 11:
Borax is an important compound of Boron.
a. The solution of borax is alkaline. Give a reason. [2]
b. Give any two uses of borax. [1]
c. Diamond has covalent bonding. Yet it has a high melting point. Give a reason. [1 ]
Question 12:
a. Give the structural formula of the following compounds:
i. 2, 4, 7 – Trimethyloctane
ii. 2 – chloro – 4 – methyl pentane [2]
b. CH3C2 or (CH3)2CH -which is more stable? Explain. [2]
c. Explain the chemistry behind crystallization. [2]
Or
a. Give the IUPAC names of the following:
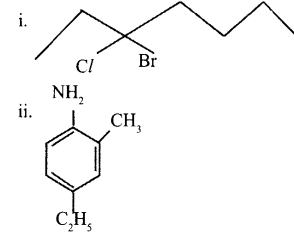
b. Which is more stable (CH3)3 C+ or CH3C+H2? Give a reason. [2]
c. Give the chemistry behind distillation under reduced pressure. [2]
Question 13:
Benzene and benzenoid compounds show aromatic character.
a. Select the aromatic compounds from the following: [1]
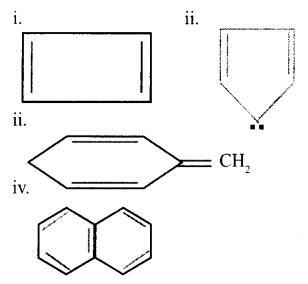
b. Suggest a method to convert Ethyne to benzene. [2]
c. Give the products formed when benzene reacts with
i. CH3 Cl/AlCl3
ii. Cl2 /hυ [2]
Question 14:
Environmental pollution is the effect of undesirable changes in surroundings that have a harmful effect on plants, animals, and human beings.
a. Explain the adverse effect of global warming. [2]
b. Choose the one which is not a component of photochemical smog.
i. NO2
ii. O3
iii. SO2