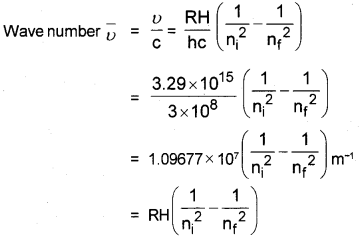Kerala Plus One Chemistry Improvement Question Paper say 2018 with Answers
| Board | SCERT |
| Class | Plus One |
| Subject | Chemistry |
| Category | Plus One Previous Year Question Papers |
Time Allowed: 2 hours
Cool off time: 15 Minutes
Maximum Marks: 60
General Instructions to Candidates:
- There is a ‘cool off time’ of 15 minutes in addition to the writing time.
- Use the ‘cool off time’ to get familiar with questions and to plan your answers.
- Read the instructions carefully.
- Read questions carefully before answering.
- Calculations, figures and graphs should be shown in the answer sheet itself.
- Malayalam version of the questions is also provided.
- Give equations wherever necessary.
- Electronic devices except non programmable calculators are not allowed in the Examination Hall.
Answer all questions from question numbers 1 to 7. Each carry 1 score. (7 × 1 = 7)
Question 1.
Name the quantum number which gives the spatial orientation of an orbital with respect to standard set of coordinate axes.
Answer:
Magnetic Quantum Number (m)
Question 2.
Among N3-, O2-, F–, Na+ and Al3+ which one has the smallest size?
Answer:
Al3+
Question 3.
Which pollutant in water causes brown mottling of teeth?
Answer:
Fluoride ion or F– ion
Question 4.
Examine the following graph and name the gas law corresponding to it.
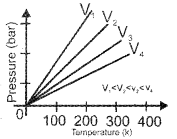
Answer:
Gay Lussac’s Law or Pressure – Temperature Law
Question 5.
If Z-axis is the internuclear axis, name the type of covalent bond formed by the overlapping of two Py orbitals.
Answer:
π bond or Pπ – Pπ bond
Question 6.
Which among the following measurements, contains the highest number of significant figures?
a) 1.123 × 10-3 kg
b) 1.2 × 10-3 kg
c) 0.123 × 103kg
d) 2 × 105 kg
Answer:
a) 1.123 × 10-3 kg
Question 7.
Write the formula of the basic structural unit of silicates.
Answer:
SiO44-
Answer any 10 questions from question numbers 8 to 20. Each carries 2 scores. (10 × 2 = 20)
Question 8.
Write any two limitations of octet rule.
Answer:
- Electrons in the outer orbital with more than 8 are also stable, eg. PCl5, SF6
- Elements with 8 electrons in the outermost orbital forms compounds, eg. Xe.
Question 9.
Draw the ‘sawhorse’ projections of the eclipsed and staggered conformations of ethane.
Answer:
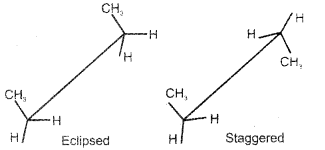
Question 10.
Give the relation between Kp and Kc for the reaction given below.
2NOCl(g) ⇌ 2NO(g) + Cl2(g)
Answer:
Kp = Kc(RT)Δn
Δn = (No. of moles of gaseous pdt – No. of moles of gaseous reactant)
(3 – 2) = 1
Kp = Kc(RT)1 ie. Kp = Kc(RT)
Question 11.
State and illustrate the law of multiple proportions.
Answer:
When two elements combine to form more than one compound the fixed mass of the element combined with the other bear a simple whole number ratio.
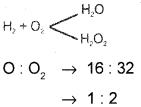
Question 12.
a) Define ‘normal boiling point’ of a liquid.
b) Give a reason for the use of pressure cooker to cook food, at high altitudes.
Answer:
a) The temperature at which the vapour pressure becomes equal to atmospheric pressure is called boiling point.
b) At high altitudes atmospheric pressure is low, therefore liquids at high altitudes boil at lower temperatures. So pressure cooker is needed for cooking at high altitudes.
Question 13.
Write two important results observed during photoelectric effect.
Answer:
- The number of ejected electrons are proportional to intensity of light.
- There is no time tag between the ejection of electron and falling of light.
Question 14.
Differentiate state functions from path functions and give one example for each.
Answer:
State functions are those functions which depend upon the initial and final state, eg. P, V, T, n, U, S, H, G. Path functions are those functions which depend upon the path. eg. Heat and work.
Question 15.
Define the terms, Biochemical Oxygen Demand (BOD) and Eutrophication.
Answer:
The amount of oxygen required by bacteria to breaking the organic matter present in a certain volume of a sample of water is called Biochemical oxygen demand.
Eutrophication: The nutrient enriched water bodies support a dense plant population which kills animal life by depriving it of oxygen and result in subsequent loss of biodiversity is known as Eutrophication.
Question 16.
Give the chemical equation for the conversion of hexane to benzene. Write the name of the process.
Answer:
n hexane → benzene process is called aromatization or reforming.
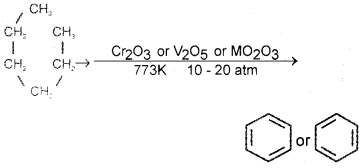
Question 17.
H2O and HSO–4 can act both as Bronsted acids and bases. For each case give the corresponding conjugate acid and conjugate base.
Answer:
H2O – Conjugate acid H3O+
Conjugate base OH–
H2SO4 – Conjugate acid HSO–4
Conjugate base SO42-
Question 18.
What is metamerism? Write the metamers of C4H10O.
Answer:
Metamerism is a type of structural isomerism seen among ketones and ethers. It is due to the difference in the number of carbon atoms on both sides of the functional group.
eg. C4H10O
CH3-O-CH2-CH2-CH3
CH3-CH2-O-CH2-CH3
Question 19.
What are zeolites? Give any two uses of zeolites.
Answer:
Zeolites are Sodium Alumino Silicate.
Uses:
- Catalyst in petrochemical industry, zsm-5 is used to convert alcohol to gasoline.
- For softening of hard water.
Question 20.
First law of thermodynamics can be stated as Δu = q + ω. How can this equation be expressed for:
a) an isothermal reversible change?
b) a process carried out at constant volume?
Answer:
q = -w
= 2.303 nRt log \(\frac{V f}{V i}\)
b) Δu = qu
Answer any 7 questions from question numbers 21 to 29. Each carries 3 scores. (7 × 3 = 21)
Question 21.
Predict the products
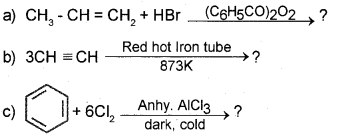
Answer:
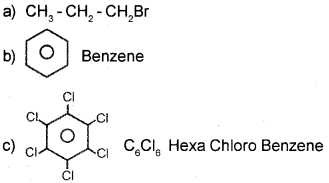
Question 22.
Redox reactions are classified into four types. Describe any three of them with suitable examples.
Answer:
Redox reactions are of four types.
i) Combination reactions A + B → C
A or B must be in elemental form. All combustion reactions are combination reactions.
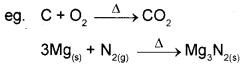
ii) Decomposition reactions are opposit of combination reactions. It involves breakdown of a compound into two or more components at least one of which must be in the elemental state.
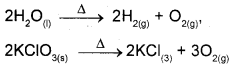
iii) In Displacement reactions an ion in a compound is replaced by an ion of another element.
x + yz → xz + y
a) Metal Dsplacement: A metal in a compound can be displaced by another metal in the uncombined state.
CuSO4(aq) + Zn(s) → Cu(s) + ZnSO4(aq)
b) Non metal displacement: The non metal displacement redox reactions include hydrogen displacement and rarely oxygen displacement.
2Na(s) + 2H2O(l) → 2NaOH(g) + H2(g)
iv) Disproportionation reaction: The same element in a compound undergoes oxidation and reduction.
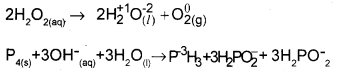
Question 23.
Enthalpies of formation of some compounds are given below:
| Compound | CO(g) | CO2(g) | N2O(g) | N2O4(g) |
| Enthalpy of formation (kJmol-1) |
Using these data, calculate the enthalpy of reaction for
N2O4(g) + 3CO(g) → N2O(g) +3CO2(g)
Answer:
ΔH Reaction
= ΣΔH0f Products – ΣΔH0f Reactants
= Σ(ΔH0fN2O(g) + 3ΔH0fCO2(g)) – Σ(ΔH0fN2O4(g) + 3ΔH0fCO(g))
= (81.0 + 3 × -393) – (9.7 + 3 × -110)
= (81 – 1179) – (9.7 – 330)
= (-1098) – (-320.3)
= -1098 + 320.3
= 777.7 kgmol-1
Question 24.
Sketch the structures of graphite and diamond. What is the impact of structure on physical properties of these allotropes?
Answer:
Structure of a diamond and graphite
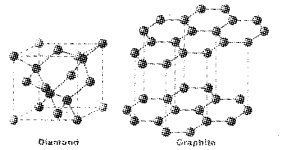
Diamond: Due to Sp3 hybridisation c forms four bonds with other four C and this makes diamond hard. There is no free electron to carry current in diamond. So it is a non conductor.
Graphite: Its structure is hexagonal layers. These layers can slip over one another, this makes graphite slippery in nature. Due to SP2 hybridisation can form only three bonds with other three carbon. One electron is free to carry current. This makes graphite a conductor.
Question 25.
Briefly explain the different types of hydrides.
Answer:
a) Ionic or saline hydrides: Hydrides formed by S block elements. These are crystalline, non volatile and non conducting in solid state, they conduct electricity in molten state.
b) Covalent or molecular Hydrides: Di hydrogen forms molecular compounds with p block elements, eg. CH4, NH3, H2O, HF
These are of three types Electron deficient, Electron precise and electron rich hydrides.
i) Electron deficient hydride – (Lewis acid) has few electrons less inwriting lewis structure, eg. group 13 elements forms only electron deficient hydride.
ii) Electron precise compounds have the required number of electrons to write the conventional Lewis structures. Group 14 elements forms such compounds.
iii) Electron rich hydrides: These compounds have excess electrons which are present as lone pairs. Elements of group 15-17 form such compounds.
c) Metallic Hydrides are formed by d block and f block elements. These are conductors of heat and electricity. Group 7, 8 and 9 do not form hydrides and it is called hydride gap.
Question 26.
Calculate the amount of CO2(g) produced by the reaction of 32g of CH4 and 32g of O2.
Answer:
CH4 + 2O2 → CO2 + 2H2O
16g CH4 + 2 × 32g O2 → 44g CO2 + 2 × 18g H2O
64g O2 → 44g CO2
32g O2 → 22g CO2
Or
64g O2 → 44g CO2
1g O2 → \(\frac{44}{64}\)gco2
32g O2 → \(\frac{44}{64}\) × 32 = 22g CO2
Question 27.
Give reasons for the following:
a) ‘O’ has lower ionization enthalpy than N and F.
b) Cl has higher negative electron gain enthalpy than F.
Answer:
0 – 1s2, 2s2, 2p4. By losing one electron oxygen will get completely half filled, electronic configuration which is extra stable.
7N – 1s2, 2s2, 2p3. completely half filled electronic configuration, extra stable.
9F – 1s2, 2s2, 2p5. One more electron is needed to get stable, octet completed electronic configuration, so I.E is very high.
Question 28.
Write the postulates of kinetic molecular theory of gases.
Answer:
- A gas contains large no. of minute particles called molecules.
- The molecules are always in random motion.
- During their motion they collide with each other and also with the walls of the container. This creates pressure.
- As the temperature increases pressure increases, kinetic energy also increases.
Question 29.
The ionization constant of nitrous acid is 4.5 × 10-4. Calculate the pH of 0.04M solution of nitrous acid in water.
(Hint: HNO2 + H2O ⇌ H3O+ + NO–2; Ka = Ca2)
Answer:
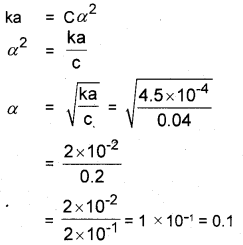
[H3O+] = Cα = 0.04 × 0.1 = 0.004
pH = – log [H3O+] = – log × 0.004
= – log 4 × 10-3
= 3 log 10 – log 4
= 3 – 0.6020 = 2.398
Answer any 3 questions from question numbers 30 to 33. Each carries 4 scores. (3 × 4 = 12)
Question 30.
Name the commercial process used to prepare sodium carbonate and write the chemical equations of the steps involved in it.
Answer:
Solvay process: Sodium Carbonate is prepared by passing C02 gas through ammoniacal brine solution. 2NH3 + H2O + CO2 → (NH4)2CO3
(NH4)2CO3+ H2O + CO2 → 2NH4 HCO3
NH4HCO3 + NaCl → NH4Cl + NaHCO3
These are heated to give Sodium Carbonate.
2NaHCO3 → Na2CO3+ CO2 + H2O
Question 31.
What is ‘sodium fusion extract’? How the presence of N, S and halogens in organic compounds are detected?
Answer:
A little amount of organic compound is mixed with metallic sodium and heated in a small ignition tube. When it becomes red hot it is plunged into little water taken in a China dish and boiled. This solution is filtered and is known as sodium fusion extract.
a) Little of the sodium fusion extract is boiled with ferrous sulphate and acidified with concentrated sulphuric acid the formation of a Prussian blue colour confirms the presence of nitrogen.
b) The sodium fusion extract is acidified with acetic acid and lead acetate is added to it. A black precipitate indicates the presence of sulphur.
Or
Sodium fusion extract with sodium nitroprusside gives a violet colour indicates the presence of sulphur.
Question 32.
The diatomic species Ne2 does not exist, but Ne–2 can exist. Explain on the basis of molecular orbital theory.
Answer:
Because the bond order for Ne2 = 0, that means Ne2 does not exist.
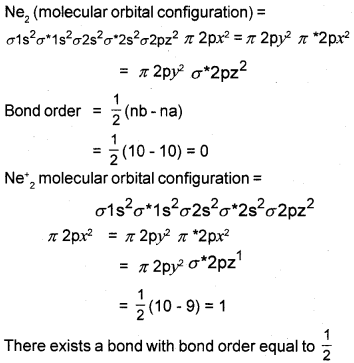
Question 33.
Explain how, the different series of lines are formed in the hydrogen spectrum. Derive an equation to find the wave number of a line in the hydrogen spectrum.
Answer:
(Hint: En = \(\frac{-R_{H}}{n^{2}}\))
RH = 2.18 × 10-18
Answer:
The electron in the hydrogen atom accepts some energy and moves to higher energy level. There it is not stable. So it emits energy and moves to lower level. This energy is shown as lines of different wave length.
In the case of H2, four spectral lines are formed.
- Lyman Series,
- Balmer Series,
- Paschen series,
- Pfund series
Derivation of Wave Number