Plus One Chemistry Chapter Wise Questions and Answers Chapter 2 Structure of Atom is part of Kerala Plus One Chemistry Chapter Wise Questions and Answers. Here we have given Plus One Chemistry Chapter Wise Questions and Answers Chapter 2 Structure of Atom.
Kerala Plus One Chemistry Chapter Wise Questions and Answers Chapter 2 Structure of Atom
Plus One Chemistry Structure of Atom One Mark Questions and Answers
Question 1.
Which of the following is not true for cathode rays?
a) They possess kinetic energy
b) They are electromagnetic waves
c) They produce heat
d) They produce mechanical pressure
Answer:
b) They are electromagnetic waves
Question 2.
The mass of the electron = ________ kg
Answer:
9.11 × 10-31 kg
Question 3.
Bohr’s orbits are called stationary states because
a) Electrons in them are stationary
b) Their orbits have fixed radii
c) The electrons in them have fixed energy
d) The protons remain in the nuclei and are stationary
Answer:
c) The electrons in them have fixed energy
Question 4.
The metal which gives photoelectrons most easily is
a) Lithium
b) Sodium
c) Calcium
d) Cesium
Answer:
d) Cesium
Question 5.
The orbitals having same energy are called ________ orbitals.
Answer:
degenerate
Question 6.
Match the following:
- Spherically symmetrical – d
- Dumb-bell – s
- Doubly dumb-bell – p
Answer:
- Spherically symmetrical – s
- Dumb-bell – p
- Doubly dumb-bell – d
Question 7.
Which of the following set of quantum numbers is correct for an electron in 4/ orbital
a) n = 4 l = 4 ml = -4 ms = +14
b) m = 4 l = 3 ml = +4 ms = +14
c) n = 4 l = 3 ml = -3 ms =-14
d) n = 4 l = 3 ml = +4 ms = -14
Answer:
c) n = 4 l = 3 ml = -3 ms = -14
Question 8.
The limiting line of the Balmer Series has the frequency of ________.
Answer:
8.23 × 1014
Question 9.
The number of orbitals and the maximum number of electrons that can be accommodated in a principal quantum level are
Answer:
n2 & 2n2
Question 10.
If the uncertainty in position and momentum of a particle like electrons are equal the uncertainty in velocity is ________
Answer:
\(\triangle V=\frac { 1 }{ 2m } \sqrt { \frac { h }{ \pi } } \)
Question 11.
The total energy of an electron in a Bohr orbit is given by ________
Answer:
\(\frac{-Z e^{2}}{8 \pi \varepsilon_{0} r}\)
Plus One Chemistry Structure of Atom Two Mark Questions and Answers
Question 1.
Match the following:
| Series | Region |
| Lyman | Infrared |
| Balmer | Ultraviolet |
| Paschen | Infrared |
| Brackett | Visible |
Answer:
| Series | Region |
| Lyman | Ultraviolet |
| Balmer | Visible |
| Paschen | Infrared |
| Brackett | Infrared |
Question 2
Of the following which is/are correct? Give justification.
a) n = 2 l = 1 m = 0 s = +½
b) n = 3 l = 3 m = 2 s =-½
c) n = 4 l = 3 m = 1 s = +½
d) n = 3 l = 2 m = 3 s = +½
Answer:
a) n = 2 f = 1 m = 0 s = +½
c) n = 4 f = 3 m=1 s= -½
Option b) is wrong because when n = 3, ^ =0, 1, 2
Option d) is wrong because when l = 2, m = -2, -1, 0, 1, 2
Question 3.
Match the following:
1. Anode rays – Nucleus
2. Cathode rays – Plum-pudding model
3. J.J Thomson – Proton
4. Thea-particle scattering experiment – Electron
Answer:
1. Anode rays – Proton
2. Cathode rays – Electron
3. J.J Thomson – Plum-pudding model
4. Thea-particle scattering experiment – Nucleus
Question 4.
Calculate the uncertainty in the determination of the velocity of a ball of mass 200 g, if the uncertainty in the determination of position is 1 A.
[h=6.626 × 10-34 J s]
Answer:

Question 5.
Which among the following sets of quantum numbers is/are not possible?
a) n = 3, l = 2, m = 0, s = +½
b) n = 2, l= 1, m = 0, s = +½
c) n = 1, l= 0, m = 0, s = -½
d) n = 4, l = 2, m = 2, s = -½
Answer:
All sets are possible.
Question 6.
1. How many sub-shells are associated with n = 4?
2. How many electrons will be present in the sub-shells having ms value of –\(\frac{1}{2}\) for n = 4?
Answer:
1. For n = 4, l can have values 0, 1, 2, 3. Thus, there are four sub-shells in n = 4 energy level.
These four sub-shells are 4s, 4p, 4d, and 4f.
2. For n = 4, the number of orbitals = (4)2 = 16.
Each orbital can have one electron with ms = –\(\frac{1}{2}\).
Thus, there are 16 electrons in sub-shells having n = 4 and ms = –\(\frac{1}{2}\)
Question 7.
i) Name the principle which restricts the pairing of electrons in degenerate orbitals. .
ii) How many electrons can be accommodated in the sub-shell having n = 4 and l = 2?
Answer:
i) Hund’s rule of maximum multiplicity,
ii) 10 electrons (4d sub-shell).
Question 8.
If the electron is to be located within 5 x 10-5A°, what will be the uncertainty in its velocity?
(Mass of the electron = 9.1 x 10-31 kg).
Answer:
According to Heisenberg’s uncertainty principle,
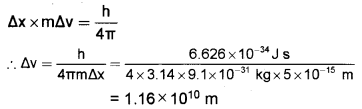
Question 9.
Nitrogen laser produces radiation at a wavelength of 337.1 nm. If the number of photons emitted is 5.6 x 1024per second, calculate the power of this laser.
Answer:
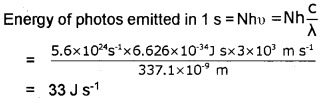
Plus One Chemistry Structure of Atom Three Mark Questions and Answers
Question 1.
Fill in the blanks suitably by studying the relationship of the given pairs:
- Lyman : Ultraviolet:: Balmer: ……………..
- s-subshell:spherical:: p-subshell: …………….
- Rydberg’s formula: \(\frac{1}{\lambda}=R\left[\frac{1}{n_{1}^{2}}-\frac{1}{n_{2}^{2}}\right]\) :: de Broglie relation:
Answer:
- Balmer: Visible
- psubshell: dumb-bell
- de Broglie relation: \(\lambda=\frac{h}{m v}\)
Question 2.
Fill in the blanks:
| Shell | n value | I value |
| K | 1 | 0 |
| L | 2 | …… |
| M | 0, 1, 2 | |
| N | 4 | ……. |
Answer:
| Shell | n-value | l-value |
| K | 1 | 0 |
| L | 2 | 0, 1 |
| M | 3 | 0, 1, 2 |
| N | 4 | 0, 1, 2, 3 |
Question 3.
The filling of electrons in the orbitals on a ground state atom is governed by three rules.
a) Which are the three rules?
b) State any one of them.
Answer:
a) 1) Aufbau principle
2) Pauli’s exclusion principle
3) Hund’s rule of maximum multiplicity
b) Hund’s rule of maximum multiplicity – electron pairing in orbitals of the same energy will not take place until each degenerate orbital of a given subshell is singly occupied.
Question 4.
During a classroom discussion, one of your friends argued that “we can’t determine both position and velocity of an electron”.
- Is it true?
- Which principle is behind your answer?
- State it.
Answer:
- Yes. It is true.
- Heisenberg’s uncertainty principle.
- Heisenberg’s uncertainty principle states that it is not possible to determine simultaneously both position and momentum of a microscopic moving particle such as electron with absolute accuracy.
Question 5.
The arguments of two students is as given:
Student 1: “We need four quantum numbers to represent an electron in a multi-electron atom.”
Student 2: “We need only first three quantum numbers to represent an electron in a multi-electron atom.”
a) Which are the four quantum numbers?
b) Who is correct? Why?
c) Write the possible four quantum numbers of the valence electron of Na atom.
Answer:
a) The four quantum numbers are:
i) Principal quantum number (n)
ii) Azimuthal quantum number (l)
iii) Magnetic quantum number (ml)
iv) Spin quantum number (ms)
b) The argument of student 1 is correct. If there are two electrons in the same subshell the first three quantum numbers become the same. Hence we need fourth quantum number to identify the electron.
c) n = 3, l = 0, m = 0, s = +½
Question 6.
a) Identify the experiment associated with the following figure:
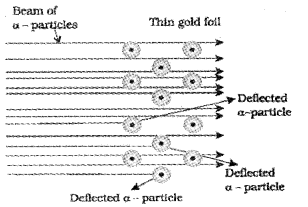
b) Explain the experiment done by Rutherford and give its observations.
c) Write the conclusions of the experiment.
Answer:
a) It is the figure of α -particle scattering experiment (gold foil experiment).
b) In this experiment, a stream of high energy α – particles from a radioactive source was directed at a thin foil of gold metal which had a circular fluorescent zinc sulphide screen around it. Whenever α-particles struck the screen, a tiny flash of light was produced at that point. The α-particles striking the gold foil were analysed. It was observed that:
- Most of the α – particles passed through gold foil undeflected.
- A small fraction of the α – particles are deflected by small angles.
- A very few α – particles(1 in 20,000) bounced back i.e., deflected by nearly 180°.
c) Rutherford drew the following conclusions regard¬ing the structure of atom from this experiment:
- Most of the space in the atom is empty as most of the α – particles passed through the foil undeflected.
- The positive charge of the atom is concentrated in a very small volume (called nucleus) that repelled and deflected the positively charged α – particles.
- Volume occupied by the nucleus is negligibly small as compared to the total volume of the atom.
Question 7.
The 4s subshell has more energy than 3p subshell.
a) Is it true? Justify your answer.
b) StateAufbau principle.
Answer:
a) Yes. For both 4s and 3p subshells the (n+l) value is 4. But 4s, with high value of ‘n’ has higher en¬ergy.
b) Aufbau principle – In the ground state of the atoms, the orbitals are filled in order of their increasing energies.
Question 8.
Two students were analysing the electronic configurations of the first 30 elements of the Periodic Table as part of an assignment. They found that two elements showed difference from other twenty eight elements.
- Which are the two elements?
- Write their electronic configurations.
- Why they show this anomalous behaviour?
Answer:
1. 24Cr and 29Cu.
2. 24Cr = 1s2 2s2 2p6 3s2 3p6 4s1 3d5 and 29Cu = 1s2 2s2 2p6 3s2 3p6 4s1 3d10
3. This is due to the fact that exactly half filled and completely filled orbitals (i.e., d5, d10) have extra stability due to symmetrical distribution of electrons and maximum exchange energy.
Question 9.
Three box diagrams of 2p3 configuration are given below:
i)![]()
- Which one is correct?
- Name the principle behind your answer.
- State the principle.
Answer:
- The correct one is (ii).
- Hund’s rule of maximum multiplicity.
- Pairing of electrons in the orbitals belonging to the same subshell does not take place until each orbital belonging to that subshell has got one electron each i.e., it is singly occupied.
Question 10.
J.J. Thomson proposed his atom model in 1898.
- Explain Thomson’s model of atom.
- Why Thomson’s atom model is called plum pudding model or watermelon model?
- What is the limitation of Thomson’s atom model?
Answer:
1. J.J. Thomson proposed that an atom possess a spherical shape in which the positive charge is uniformly distributed. The electrons are embedded into it in such a manner as to give the most stable electrostatic arrangement. The mass of the atom is assumed to be uniformly distributed over the atom. This model explained the overall neutrality of the atom.
2. Thomson’s model of atom can be visualised as a pudding or watermelon of positive charge with electrons embedded into it like the plums or seeds.
3. Thomson’s model was not consistent with the results of later experiments. It failed to explain the observations of Rutherford’s α-particle scattering experiment.
Question 11.
A student argued that the 3d orbitals will be filled only after the 4s orbital is completely filled in accordance with aufbau principle. Then another student opposed by saying that it is not true for certain elements like Cr and Cu.
a) Whose argument is correct?
b) Write electronic configurations of 24Crand 29Cu. Justify your answer.
Answer:
a) Both arguments are correct.
b) Cr and Cu have anomalous electronic configurations. This is because half-filled and completely filled sub-shells have extra stability due to the symmetrical distribution of electrons maximum exchange energy.
24Cr = 1 s2 2s2 2p6 3s2 3p6 3d5 4s1 OR [Ar]3d5 4s1 This is due to the extra stability of half filled 3d5 sub-shell.
29Cu = 1s2 2s2 2p6 3s2 3p6 3d10 4s1 OR [Ar]3d10 4s1 This is due to the extra stability of completely filled 3d10 sub-shell.
Question 12.
a) What are the atomic numbers of elements whose
outermost electronic configurations are given by
i) 3s1
ii) 3p5?
b) Which of the following are isoelectronic species?
Na+, K+, Mg2+,Ca2+, S2-,Ar
c) What will be the wavelength of a ball of mass 0.1 kg moving with a velocity of 10 ms-1? m
Answer:
a) i) 3s1-Atomic number is 11 (Na)
ii) 3p5 -Atomic number is 17 (Cl)
b) Na+, Mg2+ (both of them have same no. of electrons, i.e., 10 each)
![]()
Question 13.
Quantum numbers are a set of four numbers used to designate electron in an atom.
- How many electrons in an atom can have the following quantum numbers, n = 1, l = 0 ?
- Give the quantum numbers of the valence electron of an atom with atomic number 13.
- Draw the shape of orbital having n = 1 and l = 0.
Answer:
1. 2 electrons (i.e., 1s orbital)
2. The element with atomic number 13 is aluminium. 13Al ⇒ [Ne] 3s23p1+
The valence electron is in the 3p orbital. Hence, the quantum numbers for the valence electron are n = 3, l= 1, m = -1, 0 +1
3. It is the 1s orbital
Question 14.
a) i) What is meant by line spectra or atomic spectra?
ii) Name the series of lines in the hydrogen spectrum belonging to the visible region.
iii) What is the wavelength of light emitted when the electron in a hydrogen atom undergoes transmission from n = 4 to n = 2? (RH= 109677 cm-1)
b) State the principles/rules for filling of orbitals in atoms.
Answer:
a) i) Line spectra or atomic spectra are the spectra obtained from excited atoms due to the emission of radiation. The emitted radiation is identified by the appearance of bright lines.
ii) Balmer series
iii) n1 = 2, n2 = 4

b)
- Aufbau principle: In the ground state of the atoms, the orbitals are filled in order of their increasing energies.
- Pauli’s exclusion principle: No two electrons in an atom can have the same set of four quantum numbers.
- Hund’s rule of maximum multiplicity: Pairing of electrons in the orbitals belonging to the same subshell does not take place until each orbital belonging to that subshell has got one electron each i.e., it is singly occupied.
Question 15.
Line emission spectra are often called fingerprint of atoms.
a) Justify the above statement.
b) Yellow light emitted from a sodium lamp has a wavelength (X) of 580 nm. Calculate the frequency and wavenumber of this yellow light.
Answer:
a) Each element has a unique line emission spectrum. The characteristic lines in atomic spectra can be used in chemical analysis to identify unknown atoms in the same way as finger prints are used to identify people.
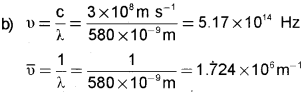
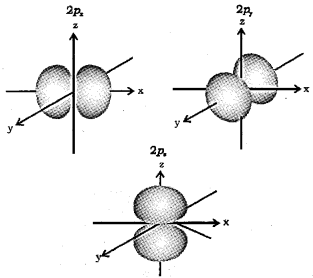
Question 16.
1. How many orbitals are possible in a p-subshell. Which are they?
2. What is the shape of p-orbital?
3. Sketch the boundary surface diagrams of 2p orbitals.
Answer:
1. Three.
These are px, py and pz orbitals.
2. Dumb-bell shaped.
3.
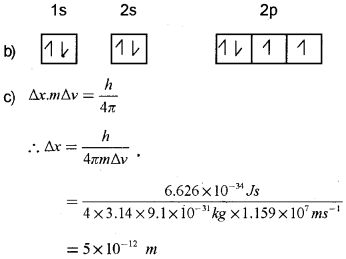
Question 17.
Electronic configuration of an element written by a student is given below:

a) Which rule is violated here?
b) Give the correct configuration.
c) What is the uncertainty in position of an electron if the uncertainty in its velocity is 1.159×107 m/s?
Answer:
a) Hund’s rule of maximum multiplicity.
Plus One Chemistry Structure of Atom Four Mark Questions and Answers
Question 1.
Bohr’s model of a hydrogen atom is a modification of
Rutherford’s model.
a) Write any two merits of Bohr’s model.
b) Write any two demerits of Bohr’s model.
Answer:
a)
- Bohr’s model could explain the stability of an atom.
- Bohr’s model could explain the atomic spectrum of hydrogen.
b)
- Failed to explain the finer details of the hydrogen atom spectrum observed by using sophisticated spectroscopic techniques.
- It could not explain the ability of atoms to form molecules by chemical bonds.
Question 2.
Consider the statement, “The two electrons of He atom have the same set of quantum numbers.”
- Do you agree?
- Name the principle applied here.
- State the principle.
- Write the all quantum numbers of outer electrons of the atom.
Answer:
- No.
- Pauli’s exclusion principle.
- No two electrons in an atom can have same set of four quantum numbers.
- Forthe1st electron: n = 2, l = 0, m = 0, s = +½.
For the 2nd electron: n = 2, l = 0, m = 0, s = -½
Question 3.
Complete the following table with respect to the va¬lence electron of each element.
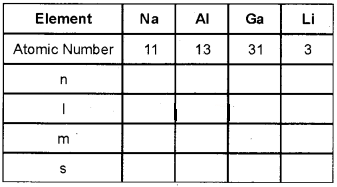
Answer:
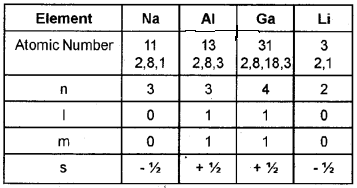
Question 4
Analyse the following figure showing transitions of electrons in the hydrogen atom.

a) Name the series (a), (b), (c), (d) and (e).
b) Mention the region of the spectrum in which each series belongs to.
c) Explain how they are obtained.
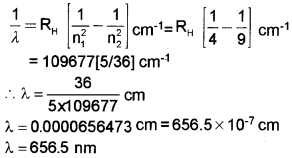
d) Calculate the wavelength of the first line of series (b).
[RH = 109677 cm-1]
Answer:
(a) = Lyman series,
(b) = Balmer series,
(c) = Paschen series,
(d) = Brackett series,
(e) = Pfund series
b)
Lyman series – UV region
Balmer series – Visible region
Paschen series – Infrared region
Brackett series – Infrared region
Pfund series – Infrared region
c) In hydrogen atom there is one electron which is present in first orbit in ground state. When energy is supplied this electron may be excited to some higher energy level. Since in a sample of hydrogen there are large number of atoms, the electrons in different atoms absorb different amounts of energies and are excited to different higher energy levels. Now, from excited states, the electron may return to ground state in one or more jumps. These different downward jumps are associated with different amounts of energies and hence result in the emission of radiations of different wavelengths which appear as different lines in the hydrogen spectrum.
Series – Obtained when electron jumps from any of the higher energy levels to
Lyman series – 1st energy level
Balmer series – 2nd energy level
Paschen series – 3rd energy level
Brackett series – 4th energy level
Pfund series – 5th energy level
d) n1 = 2, n2 = 3, RH = 109677 cm-1
Question 5.
Quantum numbers are the address of an electron in an atom. Justify the statement by explaining different quantum numbers.
Answer:
Quantum numbers are certain numbers which are used to identify an electron in an atom.
The following four quantum numbers are used for this purpose.
1. Principal quantum number (n):
It determines the size and to large extent the energy of the orbital. It also identifies the shell. The value of ‘n’ ranges from 1 to a. With increase in the value of ‘n’, the number of allowed orbitals increases and are given by ‘n2’. All the orbitals of a given value of ‘n’ constitute a single shell of atom and are represented by letters K (n = 1), L (n = 2), M (n = 3), N (n = 4) etc. The size and energy of the orbital will increase with increase of ‘n’.
2. Azimuthal/Orbital angular momentum/Subsidiary quantum number (l): It defines the three dimensional shape of the orbital. For a given value of n, l can have n values ranging from 0 to (n-1). It gives an idea regarding the subshell in which the electrons are present.
![]()
3. Magnetic quantum number (ml):
It gives information about the spatial orientation of the orbital with respect to standard set of coordinate axis. For any sub-shell, (2l+1) values of ml are possible ranging from -l to +l including zero. The permitted values of ml gives the number of orbitals in that sub-shell.
4. Spin quantum number (ms) :
It refers to the orientation of the spin of the electron. An orbital can have two electrons. If an electron is spinning in the clockwise direction, it is given a spin quantum number value of+1/2 and if an electron is spinning in the anti-clockwise direction it is given spin quantum number value of -1/2. These are called two spin states of the electron and are represented by two arrows ↑ (spin up) and ↓. (spin down). Thus, the two electrons in an orbital should have opposite spins.
Question 6.
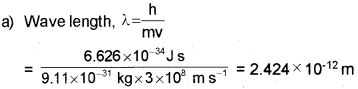
Dual nature of matter was proposed by Louis de Broglie.
a) Calculate the de Broglie wavelength associated with an electron with velocity equal to that of light.
b) State Heisenberg’s uncertainty principle and give its mathematical expression.
Answer:
b) It states that it is impossible to determine simultaneously, the exact position and exact momentum (or velocity) of an electron.
Mathematically, it can be given as in the equation
![]()
∆x ⇒ uncertainty in position of the particle
∆px ⇒ uncertainty in momentum of the particle
∆vx ⇒ uncertainty in velocity of the particle
m ⇒ mass of the particle and
h ⇒ the Planck’s constant
Question 7.
Rutherford’s atom model had strong similarity to a small scale solar system. (4)
a) What are the important features of Rutherford’s. nuclear model of atom?
b) What are the drawbacks of Rutherford’s model of atom?
Answer:
a) i) The positive charge and most of the mass of the atom is densely concentrated in extremely small region of the atom called nucleus.
ii) The nucleus is surrounded by electrons that ’ move around the nucleus with a very high speed in circular paths called orbits.
iii) Electrons and the nucleus are held together by electrostatic forces of attraction.
b) i) It failed to explain the stability of atom,
ii) It says nothing about the electronic structure of atoms.
Question 8.
a) Calculate the momentum of a particle which has de Broglie wave length of 250 pm. (4)
b) The distribution of electron into orbitals of an atom is called its electronic configuration.
i) Give the valence shell electronic configuration of chromium atom.
ii) Which among the following configurations is more.stable, d4 ord5? Justify your answer.
Answer:
a) According to the de Broglie matter wave equation
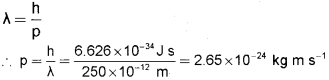
b) i) 24Cr → 1s2 2s2 2p6 3s2 3p6 3d5 4s1
ii) d5 is more stable.
The d5 configuration is half-filled. It has a symmetrical distribution of electrons and maximum exchange energy. Hence, it has extra stability compared to d4 configuration.
Question 9.
a) Name and state the principle, which restricts the maximum number of electrons in an orbital to be two.
b) Using s, p, d, f notation represent the sub-shell with the following quantum numbers.
i) n = 1, l = 0
ii) n = 4, l=3
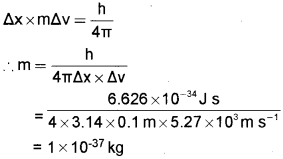
c) The uncertainty in the position and velocity of a particle are 10 cm and 5.27 × 103m/s respectively. Calculate the mass of the particle (h=6.626 × 10-34 J s).
Answer:
a) Pauli’s exclusion principle
No two electrons in an atom can have the same set of four quantum numbers.
b) i) 1s ii) 4f
c) According to Heisenberg’s uncertainty principle,
Question 10.
The photoelectric effect was first observed by H.Hertz.
a) What is the photoelectric effect?
b) What are the observations of the photoelectric effect experiment?
Answer:
a) It is the phenomenon of ejection of electrons when certain soft metals like potassium, rubidium, cae-sium, etc. are exposed to a beam of light.
b) i) The electrons are ejected from the metal surface as soon as the beam of light strikes the surface of metal.
ii) The number of electrons ejected is proportional to the intensity or brightness of light.
iii) For each metal, there is a characteristic minimum frequency (υ0), known as threshold frequency below which photoelectric effect is not observed.
Question 11.
A mathematical representation is given below:
\(\Delta x \times \Delta p_{x} \geq \frac{h}{4 \pi}\)
a) Which principle is illustrated by this equation?
b) If the position of the electron is measured within an accuracy of ±0.002 nm, calculate the uncertainty in the momentum of the electron.
c) Using s, p, d notation represent the sub-shell with the following quantum numbers:
i) n = 3 l = 2
ii) n = 5 l = 1
Answer:
a) Heisenberg’s uncertainty principle.
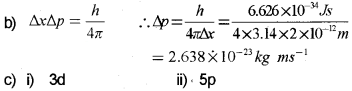
Plus One Chemistry Structure of Atom NCERT Questions and Answers
Question 1.
- Calculate the number of electrons which will together weigh one gram.
- Calculate the mass and charge of one mole of electrons.
Answer:
1. Mass of one electron = 9.11 × 10-31 kg
∴ Number of electrons in one gram = \(\frac{10^{-3} \mathrm{kg}}{9.11 \times 10^{-31} \mathrm{kg}}=1.098 \times 10^{27}\)
2. Mass of one electron = 9.11 × 10-31 kg
∴ Mass of 1 mole of electrons = 9.11 × 10-31 kg × 6.022 × 1023 = 5.486 x 10-7 kg
Charge on one electron = 1.602 × 10-19 C
∴ Charge on one mole of electrons
= (1.602 × 10-19C) × (6.022 × 1023)
= 9.65 × 104 C.
Question 2.
Write the complete symbol forthe atom with the given atomic number (Z) and atomic mass (A):
i) Z = 17, A = 35
ii) Z = 92, A = 233
iii) Z = 4, A = 9
Answer:
![]()
Question 3.
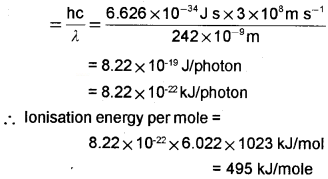
Electromagnetic radiation of wavelength 242 nm is just sufficient to ionise the sodium atom. Calculate the ionisation energy of sodium in kJ mol-1.
Answer:
Ionisation energy of sodium = Energy of one photon of radiation of wavelength.
Energy of photon = hυ
Question 4.
What is the number of photons of light with a wavelength of 4000 pm that provide 1 J energy?
Answer:
Suppose N photons of the light with wavelength 4000 pm can provide 1 J of energy.
Energy of N photons = Nhυ

Question 5.
Calculate the wavelength of an electron moving with a velocity of 2.05 × 107m S-1. (2)
Answer:
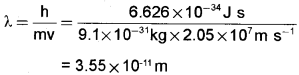
We hope the Kerala Plus One Chemistry Chapter Wise Questions and Answers Chapter 2 Structure of Atom help you. If you have any query regarding Kerala Plus One Chemistry Chapter Wise Questions and Answers Chapter 2 Structure of Atom, drop a comment below and we will get back to you at the earliest.