Plus One Chemistry Chapter Wise Previous Questions Chapter 4 Chemical Bonding and Molecular Structure is part of Kerala Plus One Chemistry Chapter Wise Previous Year Questions and Answer . Here we have given Plus One Chemistry Previous Questions Chapter 4 Chemical Bonding and Molecular Structure.
Kerala Plus One Chemistry Chapter Wise Previous Questions Chapter 4 Chemical Bonding and Molecular Structure
Question 1.
a) What do you understand by bond pair electrons and lone pair electrons? (March – 2009)
b) Explain the bond pair electrons and lone pair electrons H2O and NH3 molecules with suitable drawings.
Answer:
a) Bond pair of electrons means two atoms shares one electron each and form a bond. One bond means two electrons.
But in the case of lone pair of electrons, it will not involve in the formation of bond. But it shows bond character. In lone pair, the two electrons are owned by a single atom.
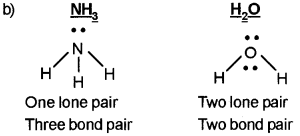
Question 2.
The stability and magnetic properties of a molecule can be well explained using the molecular orbital theory developed by F.Hund and R.S.Mulliken. (March – 2010)
a) Define bond order according to the M.O.theory.
b) Draw the energy level diagram for the formation of the O2 molecule.
c) Calculate the bond order and predict the magnetic property of the O2 molecule.
Answer:
a) Bond order is defined as one half of the difference between the number of electrons present in the bonding and the antibonding orbitals.
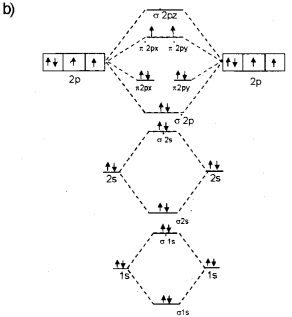
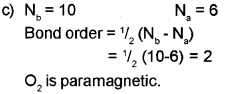
Question 3.
VSEPR theory is used to predict the shape of covalent molecules. (Say – 2010)
a) State the main postulates of VSEPR theory.
b) Based on VSEPR theory predict the shape of H2O and NH3.
Answer:
a)
- The shape of a molecule depends upon the no. of valence shell electron pairs around the central atom.
- Pairs of electron in valence shell repel one another since their electron clouds are negatively charged.
- These pairs of electron tend to occupy such positions in space that minimize repulsion and thus maximise distance between them.
b) Based on VSEPR theory shape of H2O and NH3 are given below.
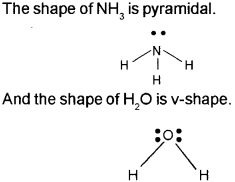
Question 4.
The attractive force which holds atoms together in a molecule is called a chemical bond. (March – 2011)
a) Explain the formation of a H2 molecule on the basis of the valence bond theory (VBT)
b) Using the molecular orbital theory (MOT), explain why a Ne2 molecule does not exist.
c) Calculate the bond order of dinitrogen (N2).
Answer:
a) Each hydrogen atom contains one electron in the 1s shell. 1s atomic orbital of first hydrogen atom overlap axially with 1s atomic orbital of second hydrogen atom to form a sigma bond. Thus in hydrogen molecule, the two hydrogen atoms are connected by a strong sigma bond.
b) Bond order of Ne2 = 1/2 (10 – 10) = 0. Since, the bond order is zero, this molecule cannot exist.
c) B.O = 1/2 (10 – 4) = 3
Question 5.
a) Hydrogen bonding plays an important role in determining the physical properties of substances. (Say – 2011)
i) Illustrate hydrogen bonding using an example.
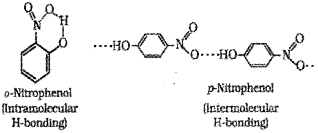
ii) Compare the boiling points of o-nitro phenol and p-Nitrophenol based on hydrogen bonding.
b) Describe the hybridization and structure of PCI5 molecule.
Answer:
a) i) Hydrogen bond can be defined as the attractive force which binds hydrogen atom of one molecule with the electronegative atom or another molecule, e.g. HF, H2O, NH3 etc.
In HF hydrogen is bonded to the electrongative element F. Hence, the electron pair shared between the two atoms moves far away from hydrogen atom. As a result, the hydrogen atom becomes highly electropositive with respect of F. Since, there is displacement of electrons towards F, hydrogen acquires fractional positive charge (δ+) while F attains fractional negative charge (δ–). This results in the formation of a polar molecule having electrostatic force of attraction.
Hδ+ – Fδ- – – – Hδ+ – Fδ- – – – Hδ- – Fδ-
The magnitude of H-bonding depends on the physical state of the compound. It is maximum in the solid-state and minimum in the gaseous state.
ii) p-Nitrophenol has higher boiling point than o- Nitrophenol.
Intermolecular hydrogen bonding increases the intermolecular attraction in p-Nitrophenol. But, intermolecular hydrogen bonding is absent in o-Nitrophenol and has intramolecular hydrogen bonding. Hence, intermolecular association through hydrogen bonding is not possible in o-Nitrophenol and has low boiling point.
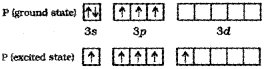
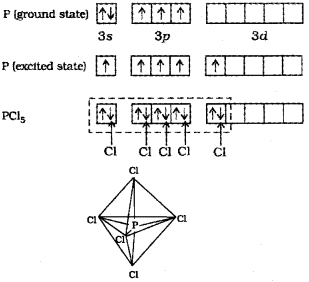
b) The ground state and the excited state outer electronic configurations of phosphorus are,
The five orbitals (i.e., one s, three p and one d orbitals) are available for hybridisation to yield a set of five sp3d hybrid orbitals which are directed towards the five comers of a trigonal bipyramidal.
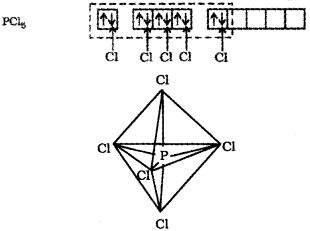
In PCI5 the five sp3d orbitals of phosphorus overlap with the singly occupied p orbitals of chlorine at-oms to form five P-CI sigma bonds. Three P-CI bond lie in one plane and make an angle of 120° with each other; these bonds are termed as equatorial bonds. The remaining two P-CI bonds-one lying above and the other lying below the equatorial plane, make an angle of 90° with the plane. These bonds are called axial bonds. As the axial bond pairs suffer more repulsive interaction from the equatorial bond pairs, therefore axial bonds have been found to be slightly longer and hence slightly weaker than the equatorial bonds; which makes PCl5 molecule more reactive.
Question 6.
Valence Bond Theory (VBT) and Molecular Orbital Theory (MOT) are the two important theories of chemical bonding. (March – 2012)
a) Out of the following, which is the hybridizatio of phosphorus in PCI5? (sp2, sp3, dsp2, sp3d)
b) Explain the geometry of the PCI5 molecule and account for its high reactivity.
c) Write the molecular orbital electronic configuration of the C2 molecule and calculate its bond order.
Answer:
a) sp3d
b) The ground state electronic configuration of P is [Ne] 3s23p3 In the excited state, one 3s-electron is excited to 3d-orbital and electronic configuration becomes 3s13p33d1. Now there five half-filled orbitals (one 3s,, three 3p and one 3d) hybridise to yield a set of five sp3d hybrid orbitals which point towards the five corners of a trigonal bipyramid.
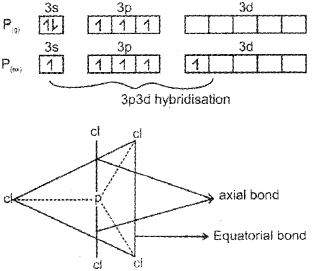
The axial bond length is larger than the equatorial bond length because of greater repulsion from other bonds ¡n axial position, consequently, pcl5 molecule is very reactive.
c) Molecular orbital configuration of C2 molecule is
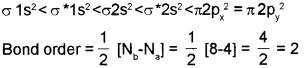
Question 7.
a) The ionic bonds have partial covalent character and the covalent bonds also show some ionic character. (Say – 2012)
¡) Explain the covalent character of Lithium chloride using Fajan’s rule.
ii) NF3 and NH3 show dipole moment. But the dipole moment of NF3 is less than that of NH3. Why?
b) The covalent bond can be explained by Molecular Orbital Theory (MOT). Using a molecular orbital diagram explain the paramagnetic nature of oxygen molecule.
Answer:
a) i) Faians Rule
In an ionic bond, some covalent character is introduced because of the tendency of the cation to polarise the anion. According to Fagan’s rule the magnitude of covalent character in the ionic bond depends upon the extent of polarisation caused by cation. In general
- Smaller the size of cation. larger its polarizing power.
- Larger the anion – more will be its plans ability.
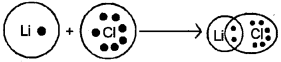
ii) Both NH3 & NF3 have pyramidal shape arid central atom has a lone pair.
In NH3 the orbital moment due to lone pair is in the same direction to the result and dipole moments of three N-H bonds and hence adds on on the resultant dipole moments.
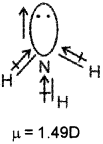
In NF3, the orbital moment due to lone pair is in the opposite direction to the resultant dipole moment of three N-F bonds hence partially cancelled by each other.
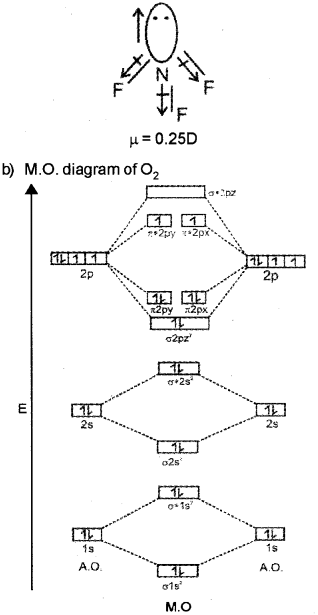
O2 molecule contain two unpaired electrons in π2py and π2px orbitalš. Hence it is paramagnetic in nature.
Question 8.
The Valence Shell Electron Pair Repulsion (VSEPR) theory helps in predicting the shapes of covalent molecules. (March – 2013)
a) Arrange the bond pair electron and lone pair electron in the decreasing order of the repulsive interactions among them.
b) A molecule of the type AB3E2 has three bond pairs and two lone pairs of electrons. Predict the most stable arrangement of electron pairs in this molecule.
c) The bond order value is an important property of a molecule. How is bond order related to bond length?
d) Write the electronic configuration of an oxygen molecule and justify its magnetic nature.
Answer:
a) Ip – Ip > Ip – bp > bp – bp
Ip – lone pair
bp – bond pair
b) A molecule of the type AB3E2 has 3- bond pairs and two lone pairs of electrons The geometry of the molecule isT-shape
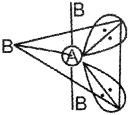
c) Bond Order (BO) is inversily proportional to bond length (B.L) ¡e B, O increases, BL. decreases.
![]()
d) Electronic configuration of an oxygen molecule is
![]()
Oxygen molecule contains two unpaired electrons in π 2px and π2py orbitals. It is paramagnetic in nature.
Question 9.
a) Only valence electrons of atoms take part in chemical combination. Draw the Lewis representation of NF3. (Say – 2013)
b) Define dipole moment. The dipole moment of BF3 is zero. Why?
c) Based on bond ofder compare the relative stability of O2 and \({ O }_{ 2 }^{ 2- }\).
Answer:
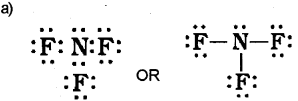
b) Dipole moment is defined as the product of the magnitude of the charge and the distance between the centres of positive and negative charge. Mathematically, Dipole moment (μ) = Charge (Q) x Distance of separation (r) BF3 has a symmetric trigonal plannar geometry in which the B-F bonds are oriented at an angle of 120° to one another. The three bond moments give a net sum of zero as the resultant of any two is equal and opposite to the third.
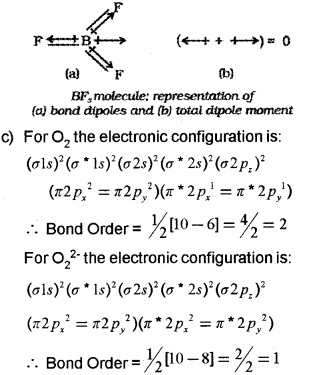
Thus, O2 with higher bond order is more stable than O22- with lower bond order.
Question 10.
a) He2 cannot exist as stable molecule. Justify this statement on the basis of bond order. (March – 2014)
b) State Fajan’s rule regarding the partial covalent character of an ionic bond.
c) Which has higher boiling point; o-nitrophenol or p-nitrophenol? Give the reason.
Answer:
a) The electronic configuration of Helium molecule is:
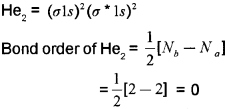
Since bond order is equal to zero, He2 molecule is unstable and does not exist.
b) The smaller the size of the cation and the larger the size of the anion, the greater the covalent charade of an ionic bond. The greater the charge on the cation, the greater the covalent character of the ionic bond.
c) p-Nitrophenol has a higher boiling point than o Nitrophenol.
Intermolecular hydrogen bonding increases the intermolecular attraction in p-Nitrophenol. But, intermolecular hydrogen bonding ¡s absent in Nitrophenol and has intramolecular hydrogen bonding. Hence, intermolecular association through hydrogen bonding is not possible in Nitrophenol and has low boiling point.
Question 11.
a) Molecular orbitais are formed by the linear combination of atomic orbitaIs (LCAO). Give the salient features of the molecular orbital theory. (August – 2014)
b) Explain sp3d hybridization with a suitable example.
OR
a) The shapes of the molecules is based on the VSEPR theory. Give the salient features of this theory.
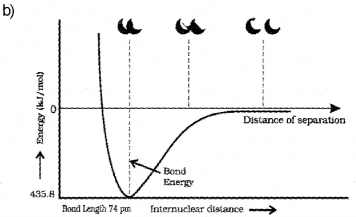
b) Draw the potential energy curve for the formation of a hydrogen molecule on the basis of the internuclear distance of the hydrogen atoms.
Answer:
a) 1) The electrons in a molecule are present in the various molecular orbitais.
2) The atomic orbitaIs or comparable energies & proper symmetry combine to form molecular orbitaIs.
3) The electron in a molecular orbital is influenced by t or more nuclei depending upon the number of atoms in the molecule.
4) The number of molecular orbital formed is equal to the number of combining atomic orbitaIs. When two atomic orbitais combine, two molecular orbitais are formed, one bonding mojecular orbital and the other antibonding molecular orbital.
5) The bonding molecular orbital has lower energy and hence greater stability than the corresponding antibonding molecular orbital.
6) The electron probability distnbution around a group of nuclei in a molecule is given by a molecular orbital.
7) The molecular orbitais are filled in accordance with the Aufbau principle obeying the Paulis exclusion principle and the Hunds rule,
b) The sp3d hybridisation is the intermixing of ones, three p and one d orbitais to yield a set of five sp3d hybrid orbitais which are directed towards the five corners of a trigonal bipyramidal with three bond angles of 1200 and two bond angles of 900. For example, in PCI5, the central atom P is sp3d hybridised. The valence electronic configuration of P in the ground state is 3s2 3p3 3d°. In the excited state a 3s electron is promoted to 3d or bital. The five half-filled orbitais intermix to form five sp3d hybrid orbitais which overlap with the singly occupied p orbita Is of chlorine atoms to form five P-Cl sigma bonds. Three P-Cl bonds lie ¡n one plane at an angle of 1200 with each other and are called equatorial bonds. The remaining two P-Cl bonds, one lying above and the other lying below the equatorial plane, make an angle of 90° with the plane and are called axial bonds.
OR
a)
- The shape of a molecule depends upon the number of valence shell electron pairs (bonded or nonbonded) around the central atom.
- Pairs of electrons ¡n the valence shell repel one another since their electron clouds are negatively charged.
- These pairs of electrons tend to occupy such positions in space that minimise repulsion and thus maximise distance between them.
- The valence shell is taken as a sphere with the electron pairs localising on the spherical surface at maximum distance from one another.
- A multiple bond is treated as ¡f it is a single electron pair and the two or three electron pairs of a multiple bond are treated as a single super pair.
- Where two or more resonance structures can represent a molecule, the VSEPR model is applicable to any such structure.
- The repulsive interaction of electron pairs decrease in the ortier lone pair (Ip) – lone pair(lp) > lone pair (Ip) – boñd pair (bp) > bond pair (bp) – bond pair.
Question 12.
The molecular orbital theory was developed by F. Hund and R.S. Mulliken. (March – 2015)
a) One-half of the difference between the number of electrons in the bonding and antibonding molecular orbitals is called …………….
ii) Predict stability and magnetic property of N2 with reasons.
OR
In order to explain the geometrical shapes of molecules, the concept of hybridization was introduced,
a) The geometry of SF6 molecule is
i) tetrahedral
ii) plannar
iii) octahedral
iv) triagonal bipyramidal
b) i) Define the term, hybridization.
ii) Explain sp3 hybridization taking methane (CH4) as an example.
Answer:
a) Bond order
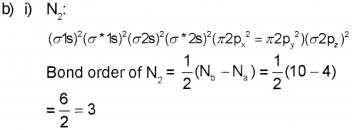
Since bond order is 3 there is a triple bond between two nitrogen atom in N, and hence it is highly stable.
Since no unpaired electron is present is N2 it is diamagnetic.
OR
a) iii) Octahedral
b) i) Hybridisation is defined as the process of intermixing of the orbitals of slightly different energies so as to redistribute their energies resulting in the formation of new set of orbitals of equivalent energies and shape,
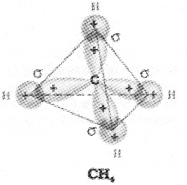
ii) In CH4 molecule there is mixing of one s-or- bital and three p-orbitals of the valence shell to form four sp3 hybrid orbitals of equivalent energies and shape. There is 25% s-character and 75% p=character in each sp3 hybrid orbital. The four sp3 hybrid orbitais so formed are directed towards the four corners of the tetrahedron. The angle between sp3 hybrid or bita.
Question 13.
The net dipole moment of a polyatomic molecule depends on the spatial arrangement of various bonds in the molecule. (Say – 2015)
The dipole moment of BF3 is zero while that of NF3 is not zero. Justify.
OR
The type of hybridization indicates the geometry of a molecule.
In water molecule, the oxygen atom is sp3 hybridzied. But water molecule has no tetrahedral geometry. Explain.
Answer:
In BF3 dueto its symmetric trigonal planar geometry the three B-F moments give a net sum of zero as the resultant of any two B – F bond moments is equal and opposite to that of the thrid.
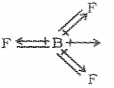
In NF3 the molecule has an unsymmetric pyramidal shape with a lone pair of electrons on nitrogen atom. Hence, the orbital dipole and bond dipoles will not cancel each other.
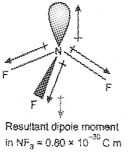
OR
In water the oxygen atom is surrounded by four sp3 hybridised orbitals of which two contain bond pairs and the other two contain lone pairs. The Ip-Ip repulsion is more than the Ip-bp repulsion which is more than bp- bp repulsion. Hence, bond angle is reduced to 104.5° from 109.5°. So the shape is distorted tetrahedral or V-shape or angular.
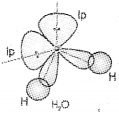
Question 14.
The formation of molecular orbitals can be described by the linear combination of atomic orbitals. (Say – 2015)
a) Which one of the following correctly represents the formation of bonding molecular orbital from the atomic orbitals having wave functions ΨA & ΨB?
i) ΨA x ΨB
ii) ΨA/ΨB
iii) ΨA + ΨB
iv) ΨA – ΨB
b) Write the electronic configuration of oxygen molecule on the basis of Molecular Orbital Theory. Justify the presence of a double bond in it and account for its paramagnetic character.
Answer:
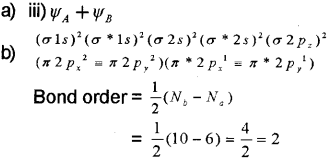
Since bond orderis two there is a double bond between two oxygen atoms in oxygen molecule.
Oxygen molecule is paramagnetic due to presence of unpaired electrons in antibonding π*2px and π*2py molecular orbitaIs,
Question 15.
a) The electronic configuration of a molecule can give information about bond order. (March – 2016)
i) Write the molecular orbital configuration of F2 molecule.
ii) Find its bond order.
b) Give any two factors influencing the formation of an ionic bond.
c) Give the shape of the following species.
i) NH4
ii) HgCI2
Answer:
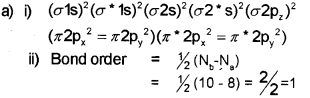
b)
- low ionisation enthalpy of the atom which fom, cation (electropositive atom/metal atom)
- highnegative electron gain enthalpy of the atom which form anion (electronegative atom/non-metal atom)
- high lattice enthalpy of the ionic compound formed (any two factors)
c) I) NHA+ -Tetrahedral OR
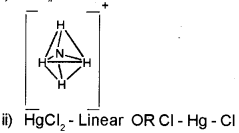
Question 16.
VSEPR theory is used to predict the shape and bond angle of molecules. (Say – 2016)
a) Write the postulates of VSEPR theory.
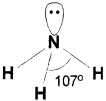
b) Explain the shape and bond angle of NH3 molecule using VSEPR theory.
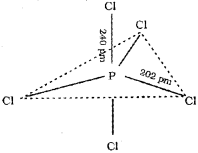
c) PCI5 molecule is unsymmetric. Why?
Answer:
a) The main postulates of VSEPR theory are as follows:
- The shape of a molecule depends upon the number of valence shell electron pairs (bonded or non-bonded) around the central atom.
- Pairs of electrons in the valence shell repel one another since their electron clouds are negatively charged.
- These pairs of electrons tend to occupy such positions in space that minimise repulsion and thus maximise distance between them.
- The valence shell is taken as a sphere with the electron pairs localising on the spherical surface at maximum distance from one another.
- A multiple bond is treated as if it is a single electron pair and the two or three electron pairs of a multiple bond are treated as a single super pair.
- Where two or more resonance structures can represent a molecule, the VSEPR model is applicable to any such structure.
- The repulsive interaction of electron pairs decrease in the order: lone pair – lone pair > lone pair-bond pair > bond pair-bond pair
b) In ammonia there are four electron pairs around the central atom nitrogen of which three are bond pairs and one is a lone pair. The three bond pair orbitals form sigma bonds with three hydrogen atoms. The lone pair is present on nitrogen atom. A tetrahedral shape is expected but due to Ip-bp repulsion which is more than bp-bp repulsion the angle between bond pairs is reduced to 107° from 109.5° and the molecule assumes a trigonal pyramidal shape.
c) The ground state electronic configuration of phosphorus (Z = 15) is [Ar] 3s2 3p3 3d°. In the excited state one of the electrons is excited to the 3Z2 orbital to give the configuration [Ar] 3s1 3p3 3dz21. In P the five orbitals (i.e., ones, three p and one d) are available for hybridisation to yield five sp3d hybrid orbitals which are directed towards the five corners of a trigonal bipyramidal. In PCIS the sp3d hybrid orbitals of P overlap with the singly occupied p orbitals (3pz) of Cl to form five P-CI sigma bonds. Three P-CI bonds lie in one plane and make an angle of 120° with each other (equatorial bonds). The remaining two P-CI bonds- one lying above and the other lying below the equatorial plane, make an angle of 90° with the plane (axial bonds). The axial bond pairs suffer more repulsive interaction from the equatorial bond pairs. Therefore, the axial bonds are slightly longer and hence slightly weaker than the equatorial bonds. Thus PCI5 molecule is unsymmetric and is more reactive.
Question 1.
The geometry of the molecule is decided by type of hybridization. (March – 2017)
a) Discuss the shape of PCI5 molecule using hybridization.
b) Give the reason for the high reactivity of PCI5.
c) isoelectronic species have the same bond order. Among the following, choose the pair having same bond order.
C\(\bar{N}\), \(\bar{O}\), NO, CN
Answer:
a) The ground state electronic configuration of phosphorus (Z=15) is [Ar) 32 3p3 3d°. In the excited state one of the 3dz2 electrons is excited to the 3dz2 orbital to give the configuration [Ar) 32 3p3 3dz2. In P the five orbitaIs (i.e., one s, three p and one d) are available for hybrid isation to yield five sp3d hybrid orbitaIs which are directed towards the five corners of a tngonal bipyramidal. In PCI5 the sp3d hybrid orbitais of P overlap with the singly occupied p orbitais (3pz) of Cito form five P-CI sigma bonds. Three P-CI bands lie in one plane and make an angle of 1 20°with each other (equa torial bonds). The remaining two P-CI bonds-one lying above and the other lying below the equatorial plane, make an angle of 900 with the plane (axial bonds).
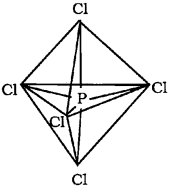
b) In PCI5 the axial bond pairs suffer more repulsive interaction from the equatorial bond pairs. Therefore, the axial bonds are slightly longer and hence slightly weakerthan the equatorial bonds. This makes PCI5 molecule more reactive.
c) CN and NO+
(Explanation: both have 14 electrons and bond order of 3)
We hope the Kerala Plus One Chemistry Chapter Wise Previous Questions Chapter 4 Chemical Bonding and Molecular Structure help you. If you have any query regarding Kerala Plus One Chemistry Chapter Wise Previous Questions Chapter 4 Chemical Bonding and Molecular Structure, drop a comment below and we will get back to you at the earliest.