Kerala SSLC Chemistry Model Question Papers with Answers Paper 3 are part of Kerala SSLC Chemistry Previous Year Question Papers with Answers. Here we have given Kerala SSLC Chemistry Model Question Papers with Answers Paper 3.
| Board | SCERT |
| Class | SSLC Class 10 |
| Subject | Chemistry |
| Category | Kerala SSLC Previous Question Papers |
Kerala SSLC Chemistry Model Question Papers with Answers Paper 3 Free Download English Medium
Time Allowed: 1 1/2 Hours
Maximum Marks: 40
Instructions
- First 15 minutes is cool-off time.
- Read all questions carefully.
- Questions with scores 1, 2, 3 and 4 are categorised separately.
- 5 questions are given in each category. Answer any 4 questions from each category.
- Answer each question by keeping time.
Attempt any 4 questions from 1 to 5 (Each questions carries 1 score)
Question 1:
Iron is extracted using the blast furnace. Name one chief ore of iron.
Question 2:
The method used for the conversion of carbonate ore to oxide ore ______
Question 3:
Find the relation and fill in the blank
Making lenses : Flint glass
Making laboratory equipment: _____
Question 4:
Certain chemical equations indicating reversible reactions are given below.
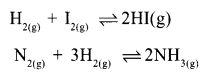
Which of the above reaction has no effort on pressure changes?
Question 5:
Identify the ionic molecule from the following given below,
a. MgCl2
b. Cl2
c. HF
d. F2
Answer any 4 questions from 6 to 10 (each questions carries 2 score)
Question 6:
Complete the table
(Atomic mass : Na – 23, 0-16)
| Substance | Number of molecules | Number of atoms | Mass |
| Sodium | 6.022 x 10²³ | (a) | 23 g |
| Oxygen | (b) | 2 x 6.022 x 10²³ | fc) |
| Ozone | 6.022 x 10²³ | (d) | 48 g |
Question 7:
The compounds of transition metals are usually used for giving colour to glass. Write the colour obtained by adding the following compounds
a. Ferric ion
b. Manganese dioxide
c. Cobalt oxide
d. Chromium ion
Question 8:
The ore of aluminium is bauxite.
a. Which solution is used for the concentration of bauxite?
b. Why this solution is selected for the process?
Question 9:
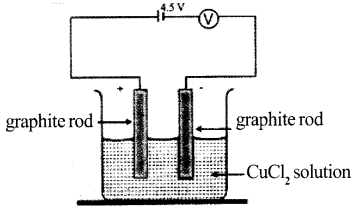
Analyze the picture. Write the balanced equations of the reactions taking place at negative electrode and positive electrode.
Answer any 4 questions from 10 to 15 (3 scores each)
Question 10:
Analyse the picture and write answer to the questions given below.
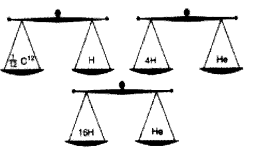
(Hint: Atomic mass is expressed in terms of the mass of \(\frac { 1 }{ 12 }\) of a carbon -12 atom)
a. Which is the possible mass of a helim atom?
![]()
b. How many times will be the mass of an oxygen atom greater than the mass of \(\frac { 1 }{ 12 }\)C12 atom?
Question 11:
Complete the following equations. (3)
a. Na2S2O3 (aq) + 2HCl(aq) →
b. MgCO3 (s) + 2HCl(aq) →
c. Zn(s)+2HCl(aq) →
Question 12:
a. Complete the following equation.
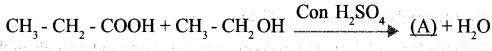
Question 13:
Marble reacts with dilute HCl according to the equation.
CaCO3+ 2HCl → CaCl2+H2O + CO2
a. Calculate the number of grams of water produced when two moles of CaCO3 reacts with excess HCl.
b. What volume of CO2 gas is liberated at STP when 500g of CaCO3 reacts with excess HCl.
(Atomic mass Ca – 40, Cl – 35.5, O -16. C -12, H -1)
Question 14:
Metallurgy involves all the processes to the separation of a pure metal from its ore.
a. Distinguish a mineral from an ore.
b. What type of ores are usually concentrated by froth floatation process. Give an example.
Question 15:
The chemical reactions of various Galvanic cells are given as incomplete in the table. Complete them.
| Cell | Chemical reaction | Redox Reaction | |
| Anode | Cathode | ||
| Zn – Cu | (a) | Cu2++2e → Cu | (b) |
| Fe – Ag | (c) | (d) | Fe++2Ag+ → Fe++2Ag |
| Mg – Pb | Mg → Mg2+ + 2e– | (e) | (f) |
Answer any 4 from questions 16 to 20. Each question carries 4 score
Question 16:
Analyze the equation given below.
Ca(OH)2(aq) + 2HCl(aq) → CaCl2(aq) + 2H2O(ℓ)
a. Calculate the Gram Molecular Weight (GMM) of Ca(OH)2.
b. Calculate the mass of HCI in grams needed to neutralize 500 g of Ca(OH)2.
c. How many grams of water will be produced during the reaction given in question(b)?
[Ca – 40, O – 16, H – 1, Cl -35.5]
Question 17:
The ore of iron is hematite.
a. What are the substances added to the blast furnace along with hematite during the extraction of iron?
b. Explain the uses of these substances with the equations.
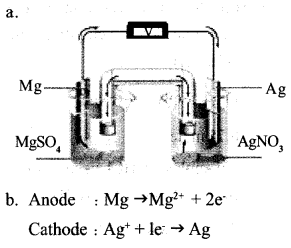
Question 18:
a. Draw the pictorial representation of Mg – Ag cell.
b. Write the equations of the reaction taking place at anode and cathode of this cell.
Question 19:
TheIUPAC name of a compound is given below. 2,2-Dimethylpropane
a. Write the structure of this compound.
b. Write all the possible isomers of this compound.
c. Write the IUPAC name of these compounds.
Question 20:
The figure of an incomplete periodic table is given below?
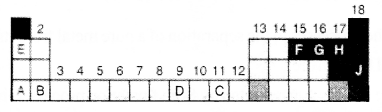
a. Which one of these elements show – 2 oxidation state?
b. Which of these elements have 3 electrons in their outermost p subshell?
c. Which element has the highest atomic radius?
d. Which of these element show variable oxidation state?
ANSWERS
Answer 1:
Haematite (Fe2O3)
Answer 2:
Calcination
Answer 3:
Borosilicate glass
Answer 4:
H2 + I2 ⇌ 2HI
Answer 5:
MgCl2
Answer 6:
a. 6.022 x 1023
b. 6.022 x 1023
c. 32 g
d. 3 x 6.022 x 1023
Answer 7:
a. Yellow
b. Purple
c. Blue
d. Green
Answer 8:
a. Hot NaOH solution.
b. Bauxite alone dissolves in hot NaOH solution. The insoluble impurities can be removed easily.
Answer 9:
Positive electrode:
2Cl → Cl2 + 2e–
Positive electrode:
Cu2- + 2e–→ Cu
Answer 10:
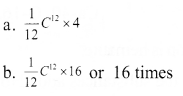
Answer 11:
a. Na2S2O3(aq)+2HCl(aq) → 2NaCl (aq) + H2O (ℓ) + SO2(g) + S(s)
Answer 12:
a. A → CH3 – CH2 – COOH2CH3
b. Esters
c. Esters have the natural fragrance of flowers and fruits.
Answer 13:
a. When 1 mole of CaCO, reacts with HCl
1 Mole of H2O is obtained
When 2 moles of CaCO3 reacts with HCl
2 Moles of H2O is obtained
2 Moles of H2O= 36gm
b. Molecular mass of CaCO3
= 40+ 12 + 48 = 100
When 100g of CaCO3 reacts with
HCl 1 mole of CO2 is produced.
When 500g of CaCO3 reacts 5
moles of CO2 is produced.
Volume of 5 moles of CO2 at STP
= 22.4 x 5 = 112l
Answer 14:
a. Elements or their compounds occuring naturally are called Minerals. The mineral from which the metal can be extracted easily and economically is called the Ore of the metal.
b. When the ore has low density and impurities have high density froth floatation method is used.
Answer 15:
![]()
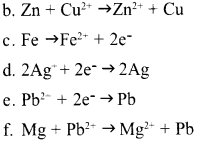
Answer 16:
a. GMM ofCa(OH)2
= 40+16 x 2 + 2
= 40+32+2 = 74g.
b. According to this equation, 2 mole of HCl are needed to neutralize 1 mole of Ca(OH)2
500g Ca(OH)2 \(\frac { 500 }{ 74 }\) mole
= 6.75 mole
no.of moles of HCl needed to neutralize 6.75 mole of Ca(OH)2
= 2 x 6.75 mole = 13.5 mole
GMM of HCl = 1+35.5 g
= 36.5 g
Mass of HCl = 13.5 x GMM of HCl
= 13.5 x 36.5 g = 492.75 g
c. According to the equation, during the neutralization 1 mole of Ca(OH)2 ,
2 mole of water is formed.
The no.mole of H2O produced when 6.75 mole of Ca(OH)2 is neutralized.
= 2 x 6.75 mole = 13.5mole
GMM of water = 2+16 g = 18 g
Mass of H,0 = 13.5 x 18g = 243 g
Answer 17:
a. Coke (C)
Lime stone CaCO3
b. Coke react with oxygen in the furnace to form CO gas. This CO reduces iron oxide to iron.
C + O2 → CO2 C + CO2 → 2CO
Fe2O3+3CO → 2Fe + 3CO2
Lime stone decomposes at high temperature to form CaO (quick lime).
This react with SiO2, the gangue in the ore of iron, forming slag
CaCO3 → CaO + CO2;
CaO + SiO2 → CaSiO3
Answer 18:
Answer 19:
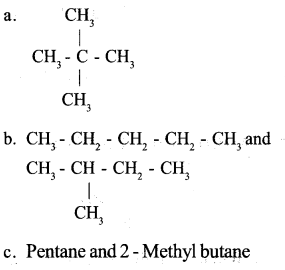
Answer 20:
a. G
b. F
c. The element having highest atomic radius -A
The element having lowest atomic radius – H
d. -D,C
We hope the Kerala SSLC Chemistry Model Question Papers with Answers Paper 3 help you. If you have any query regarding Kerala SSLC Chemistry Model Question Papers with Answers Paper 3, drop a comment below and we will get back to you at the earliest.