What is meant by Colloidal Solution
Colloids :
A colloid is a kind of solution in which the size of solute particles is intermediate between those in true solutions and those in suspensions. The size of solute particles in a colloids is bigger than that of a true solution but smaller than those of a suspension.
Dispersed particles
The solute particles are also called ‘dispersed particles’
Dispersion medium
Solvents are also known as dispersion medium.
Solution, suspensions and colloids differ in the size of solute particles, the size of particles being minimum in solutions and maximum in suspensions.
Properties of colloidal solutions
- Heterogeneous Nature : A colloidal solution is heterogeneous in nature. It consists of two phases : dispersed phase and dispersion medium.
- Filtrability : The size of the colloidal particles is less than the pores of a filter paper, and, therefore, they easily pass through a filter paper. Colloidal particles however, cannot pass through the parchment paper or an animal membrane or ultra-filter.
- Tyndall Effect : When a strong beam of light is passed through a colloidal solution placed in dark place, the path of the beam gets illuminated by a bluish light . This phenomenon is called Tyndall effect. The phenomenon is due to the scattering of light by the colloidal particles. The same phenomenon is noticed when a beam of sunlight enters a dark room through a small slit, due to scattering of light by dust particles in the air.
- Visibility : Colloidal particles are too small to be seen by the naked eye. They however, scatter light and become visible when viewed through an ultra microscope.
- Brownian movement : When colloidal particles are seen under an ultra microscope, the particles are found to be in constant motion in zig-zag path in all possible directions. This zig-zag motion of colloidal particles is called Brownian movement. The movement of the particles is due to the collisions with the molecules of the dispersion medium.
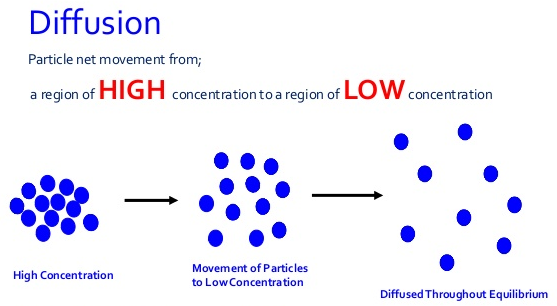
- Diffusion : Colloidal particles diffuse from a region of higher concentration to that of lower concentration. However, because of their bigger sizes colloidal particles move slowly and hence diffuse at slower rate.
- Sedimentation or settling : Under the influence of gravity, the solute particles tend to settle down very slowly. This rate of settling down or sedimentation can be accelerated by the use of high speed centrifuge called ultra-centrifuge.
Classification of colloids
Colloids are classified according to the physical state of dispersed phase (solute) and the dispersion medium (solvent). Most of the colloids can be classified into the following seven groups.
- Sol : Sol is a colloid in which tiny solid particles are dispersed in a liquid medium.
Ex. Ink, Soap solution, starch solution and most paints. - Solid sol : Solid sol is a colloid in which solid particles are dispersed in a solid medium.
Ex. Coloured gemstones (like ruby glass). - Aerosol : An aerosol is a colloid in which a solid or liquid is dispersed in a gas (including air).
Ex. The examples of aerosols in which a solid is dispersed in a gas are : Smoke (which is soot in air) and Automobile exhausts. The examples of aerosols in which a liquid is dispersed in a gas are : Hairspray, Fog, Mist and clouds. - Emulsion : An emulsion is a colloid in which minute droplets of one liquid are dispersed in another liquid which is not miscible with it.
Ex. Milk, butter and Face cream. - Foam : The foam is a colloid in which a gas is dispersed in a liquid medium.
Ex. Fire-extinguisher foam ; Soap bubbles, shaving cream and Beer foam. - Solid foam : The solid foam is a colloid in which a gas is dispersed in a solid medium.
Ex. Insulating foam, foam rubber and Sponge. - Gel : The gel is a semi-solid colloid in which there is a continuous network of solid particles dispersed in a liquid.
Ex. Jellies and Gelating.
Concentration of solution
Dilute solution : The solution having small amount of solute is said to have low concentration. it is known as a dilute solution.
Concentrated solution : The solution having a large amount of solute is said to be of high concentration. It is known as a concentrated solution. The concentration of a solution is amount of solute present in a given quantity of the solution. The most common way of expressing the concentration of a solution is the ‘percentage method’.
Ex. A 10 per cent solution of common salt means that 10 grams of common salt are present in 100 grams of the solution.
We can calculate the concentration of a solution in terms of mass percentage of solute by using the following formula.
concentration of solution = \(\frac { Massofsolute }{ Massofsolution }\times 100\)
The mass of solution is equal to the mass of solute plus the mass of solvent. That is :
Mass of solution = Mass of solute + Mass of solvent
So, we can obtain the mass of solution by adding the mass of solute and the mass of solvent.
In the above given example :
Mass of solute (salt) = 10 g
And, Mass of solvent (water) = 90 g
So, Mass of solution = Mass of solute + Mass of solvent
= 10 + 90 = 100 g
Now, putting these values of ‘mass of solute’ and ‘mass of solution’ in the above formula, we get :
Concentration of solution = \(\frac { 10 }{ 100 }\times 100\)
= 10 per cent (by mass)
The case of a liquid solute dissolved in a liquid solvent : In the case of a liquid solute dissolved in a liquid solvent : The concentration of a solution is defined as the volume of solute in millilitres present in 100 millilitres of the solution.
Ex. A 20 per cent solution of alcohol means that 20 millilitres of alcohol are present in 100 millilitres of solution.
Concentration of solution = \(\frac { Volume of solute }{ Volume of solution }\times 100\)
Solubility
The maximum amount of a solute which can be dissolved in 1 litre of a solution at a specified temperature is known as the solubility of that solute in that solvent (at that temperature).
Effect of temperature and pressure on solubility
- The solubility of solids in liquids usually increases on increasing the temperature; and decreases on decreasing the temperature.
- The solubility of solids in liquids remains unaffected by the changes in pressure.
- The solubility of gases in liquids usually decreases on increasing the temperature; and increases on decreasing the temperature.
- The solubility of gases in liquids increases on increasing the pressure; and decreases on decreasing the pressure.
 Diffusion in gases
Diffusion in gases