What happens to the Wax in a Candle
Combustion of a Wax Candle
If you observe a candle flame closely, you will notice the following.
- The wick burns and it stands in a pool of liquid wax.
- There is a small portion of unburnt wick between the flame and the liquid wax.
- The liquid wax is trapped in a ‘cup’ of solid wax.
- The liquid or solid wax never catches fire.
We can see that the wick is burning. But it cannot be the only substance that is burning as the candle gets smaller as it burns. So, what is burning in addition to the wick? It is the wax vapours that burn.
If a lit match is brought a little above a candle wick immediately after the candle has been blown out, it is noticed that the flame from the match jumps the gap and reignites the wick. This happens because the wax vapours rise from the wick immediately after the candle is blown out and the burning match reignites them. From this, we conclude the following.
- It is only the wax vapours that burn. Neither liquid wax nor solid wax burns.
- When a candle wick is lit, the heat produced from the flame melts the wax.
- The wick soaks or absorbs the molten wax.
- The heat of the flame vaporizes the molten wax in the wick.
- The wax vapours burn in the flame. This process continues till the entire wax is consumed or the candle is extinguished.
Activity
Aim: To prove that when a candle burns, it is the wax vapours that burn and not the liquid wax.
Materials needed: A candle and a matchbox
Method:
1. Light the candle using a match.
2. Blow out the candle and immediately bring a burning matchstick near the smoke. In case the smoke is not visible, hold the lit match above the wick.
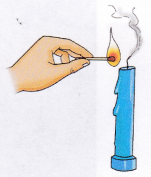
Observation: The flame from the match reignites the wick.
Conclusion: When a candle is lit, it is the wax vapours that burn, not the liquid wax.
Aim: To prove that when a candle is lit, the liquid wax absorbed by the wick is converted into vapours.
Materials needed: A candle, a matchbox, and a pair of tongs or tweezers
Method:
1. Light the candle using a match.
2. Take a pair of tongs or tweezers and pinch the wick tightly just below the flame for some time.
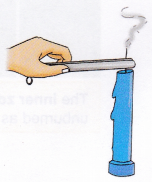
Observation: The flame goes out.
Conclusion: By pinching the wick, the absorption of liquid wax by the wick stops, which cuts off the flame’s fuel supply and it extinguishes.
Aim: To prove that carbon dioxide is produced on burning candle wax.
Materials needed: A candle, a matchbox, a glass jar, aluminium foil, and lime water solution
Method:
1. Fix a candle into the glass jar.
2. Light the candle using a matchstick.
3. Cover the beaker with a piece of aluminium foil to cut down the air supply.
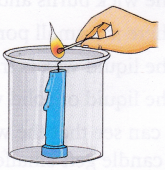
4. When the candle goes out, slowly and carefully remove some portion of the aluminium foil and take out the candle from the beaker, trying not to disturb the contents of the beaker. Pour the lime water solution down the side of the beaker and swirl it.
Observation: Lime water turns milky.
Conclusion: Combustion of candle wax produces carbon dioxide gas, which reacts with lime water to produce insoluble calcium carbonate.
Aim: To prove that water vapour is produced on burning candle wax Materials needed; A lit candle, spoon, ice-cold water, and paper napkin
Method:
1. Dip a spoon in ice-cold water to cool it to below room temperature (figure A).
2. Dry the spoon carefully with the paper napkin and hold it above the flame of the lit candle for a second or two (figure B).
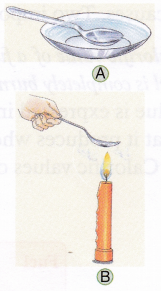
Observation: Water vapour has condensed on the cold spoon.
Conclusion: Water vapour is produced on burning candle wax.