Explain Bohr Bury rules for Distribution of Electrons into Different Shells
Electron distribution
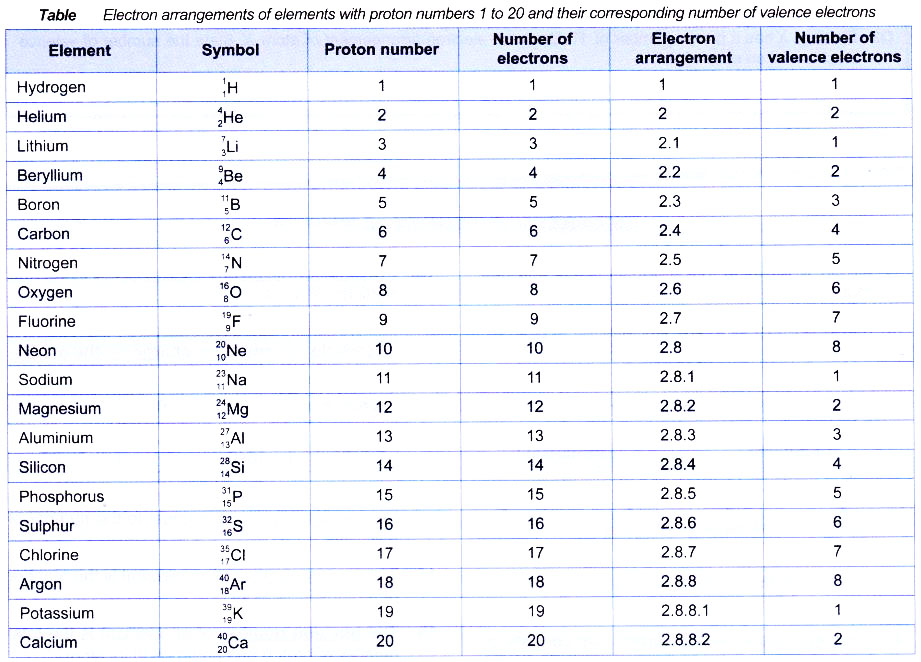
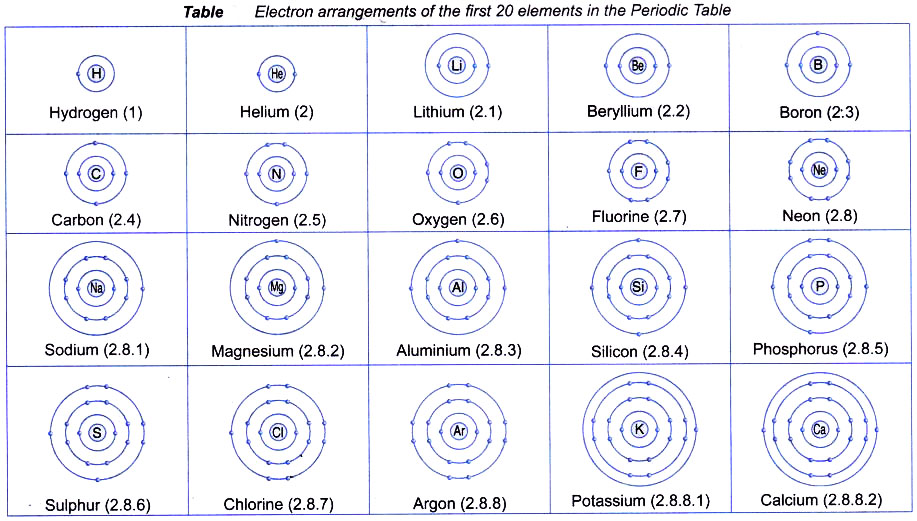
The distribution of electrons in different orbits or shells is governed by a scheme known as Bohr bury scheme.The arrangement of electrons in various energy levels of an atom is known as the electronic configuration of the atom. According to this scheme.
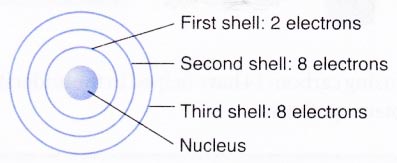
- The electrons are arranged around the nucleus in different energy levels or energy shells. The electrons first occupy the shell with the lowest energy i.e., closest to the nucleus.
- The first or the innermost energy shell (K or n = 1) can take only two electrons.
- The second shell (L or n = 2) can contain upto 8 electrons.
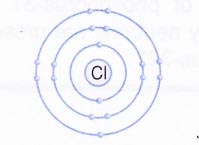
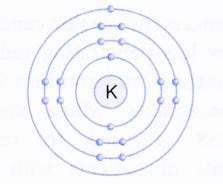
- From third shell (M or n = 3) onwards, the shells become bigger. The third shell can accommodate as many as 18 electrons. In general, the maximum number of electrons that can be present in any shell is 2n2 where n is the number of energy shell. Thus, the first orbit (n = 1, known as K shell) can contain 2 × 12 = 2 electrons, the second orbit (n = 2, known as L shell) can contain 2 × 22 = 8 electrons.
Maximum No. of electrons in different orbits
Orbit | Value of n | Maximum no. of electrons in the orbit |
K | 1 | 2 × 12 = 2 |
| L | 2 | 2 × 22 = 8 |
M | 3 | 2 × 32 = 18 |
| N | 4 | 2 × 42 = 32 |
The outermost shell of an atom cannot have more than 8 electrons and the shell next to the outermost shell cannot have more than 18 electrons.
People also ask
- How would you describe the Structure of an Atom
- What was Rutherford’s Original Hypothesis
- What did Bohr Contribute to the Theory of an Atom
- What are the Characteristics of Electron, Proton and Neutron
- What are the Isotopes, Isobars and Isotones of an Element
- What did Dalton Contribute to the Understanding of the Atom
- What is the Definition of Atom and Molecule
- What is Atomic Mass
- How has the Model of the Atom Changed Over the Years?
Valence shell
The outermost orbit of an atom is called its valence shell.
Valence electrons
The electrons present in the outermost orbit are called valence electrons.
Example: The electronic configuration of lithium is 2, 1 or it may be represented as :
K L
2 1
the outermost orbit is L shell and the number of electrons in its valence shell is one. Only the valence electrons of an atom take part in chemical reactions because they have more energy than all the inner electrons of the atom.
Valency :
Valency of an element is the combining capacity of the atoms of the element with atoms of the same or different elements.
Types of valency
There are two types of valency : Electrovalency and covalency. If an element combines by the loss or gain of electrons to form electrovalent compounds (or ionic compounds), its valency is known as electrovalency, and if an element combines by the sharing of electrons to form covalent compounds (or molecular compounds), its valency is known as covalency.
- Electrovalency : In the formation of an electrovalent compound (or ionic compound), the number of electrons lost or gained by one atom of an element to achieve the nearest inert gas electron configuration is known as its electrovalency. The elements which lose electrons form positive ions, so they have positive electrovalency. So they have positive electrovalency. The elements which gain electrons form negative ions, so they have negative electrovalency.
- Covalency : In the formation of a covalent compound the number of electrons shared by one atom of an element to achieve the nearest inert gas electron configuration is known as its covalency. If an atom shares 1 electron, its covalency will be 1.