How can we Separate a Mixture of Two Solids
Many of the materials around us are mixtures. These mixtures have two or more than two substances mixed in them. It may not be possible to use a mixture as such in homes and in industries. We may require only one (or two) separate constituents of a mixture for our use. So, we have to separate the various mixtures into their individual constituents to make them useful in our daily life.
Separation of mixture of two solids
All the mixtures containing two solid substance can be separated by one of the following methods:
1. Separation by a suitable solvent : In some cases, one constituent of a mixture is soluble in a particular liquid solvent whereas the other constituent is insoluble in it. This difference in the solubilities of the constituents of a mixture can be used to separate them.
Ex. Sugar is soluble in water whereas sand is insoluble in it, so a mixture of sugar and sand can be separated by using water as solvent.
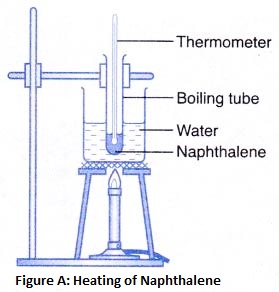
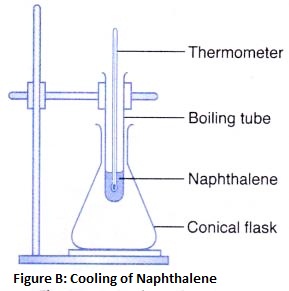
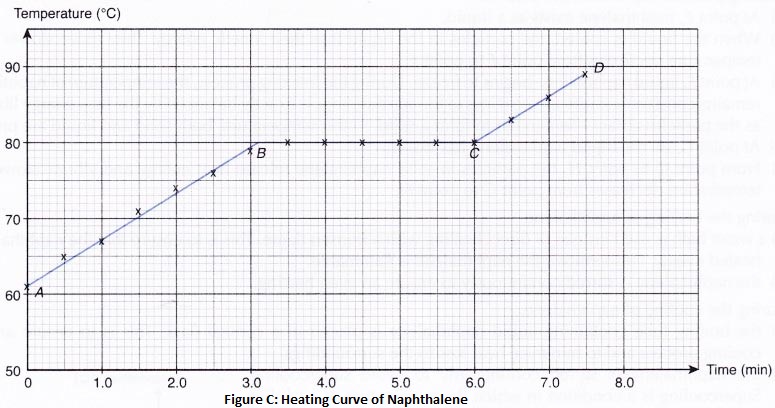
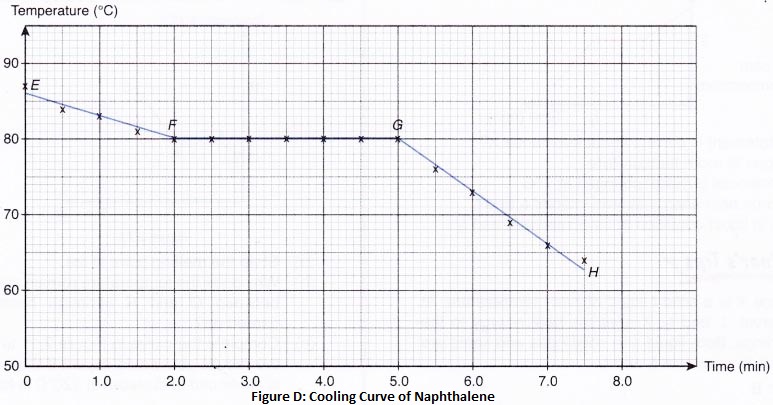
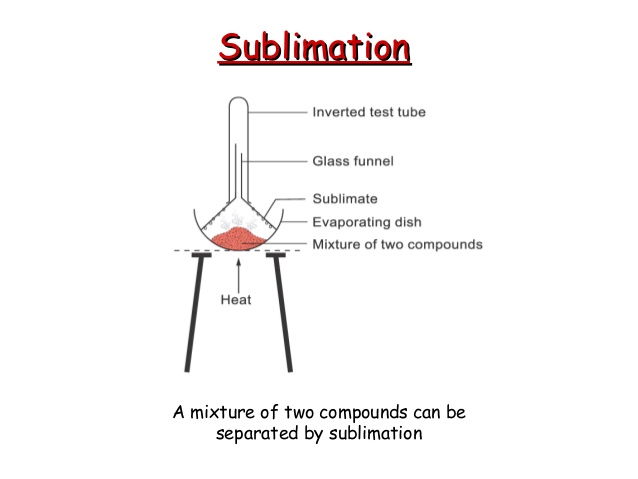
2. Separation by sublimation : The changing of a solid directly into vapours on heating, and of vapours into solid on cooling is called sublimation. The solid substance which undergoes sublimation is said to ‘sublime’. The process of sublimation is used to separated those substances from a mixture which sublime on heating. The solid substance obtained by cooling the vapour is known as sublimate.
Ex. Ammonium chloride, Iodine, Camphor,. can be separated from a mixture by sublimation.
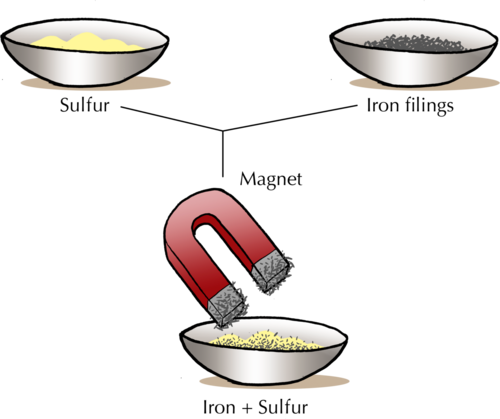
3. Separation by a magnet : Iron is attracted by a magnet. This property of iron is used to separate it from a mixture. So, if a mixture contains iron as one of the constituents, it can be separated by using a magnet.
Ex. A mixture of iron filings and sulphur power can be separated by using a magnet. This is because iron filings are attracted by a magnet but sulphur is not attracted by a magnet.
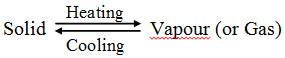 The solid substance which undergoes sublimation is said to ‘sublime’. the solid obtained by cooling the vapours of the solid is called a ‘sublimate’.
The solid substance which undergoes sublimation is said to ‘sublime’. the solid obtained by cooling the vapours of the solid is called a ‘sublimate’.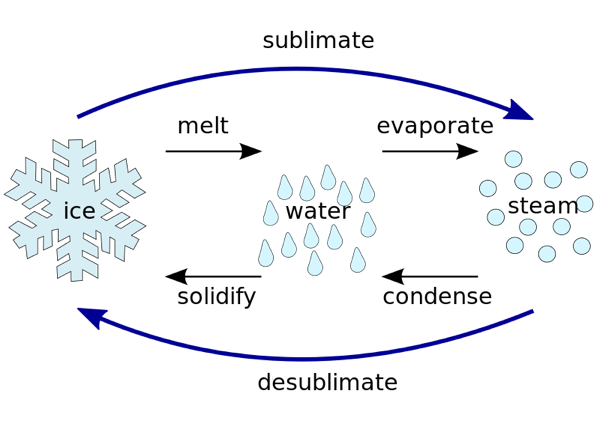 Ex. When solid ammonium chloride is heated, it directly changes into ammonium chloride vapour. And when hot Ammonium chloride vapour is cooled, it directly changes into solid ammonium chloride. Ammonium chloride, Iodine, Camphor, Naphthalene and Anthracene.
Ex. When solid ammonium chloride is heated, it directly changes into ammonium chloride vapour. And when hot Ammonium chloride vapour is cooled, it directly changes into solid ammonium chloride. Ammonium chloride, Iodine, Camphor, Naphthalene and Anthracene.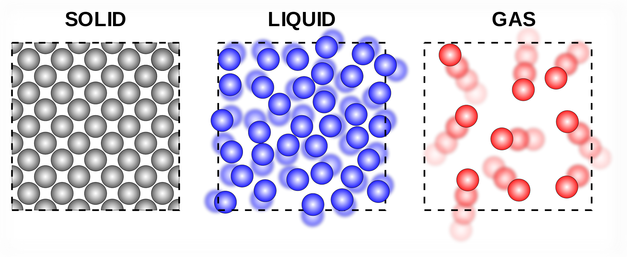
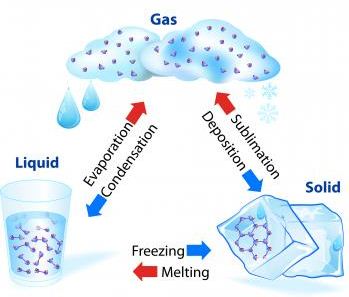 Boiling/Evaporation:
Boiling/Evaporation: