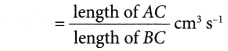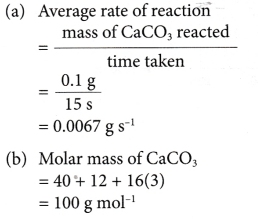Explain the effect of concentration on the rate of reaction?
Effect of concentration on the rate of reaction:
- When the concentration of a reactant increases, the rate of reaction also increases.
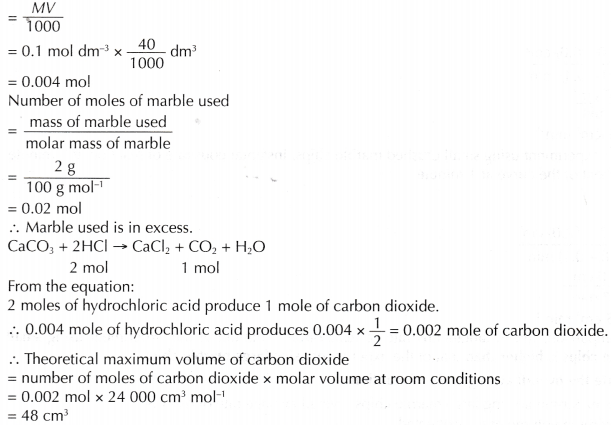
- (a) For example, two sets of experiments are carried out using the reacting conditions below:
Set I: 1 g of zinc powder and 20 cm3 of 0.4 mol dm–3 hydrochloric acid at room temperature.
Set II: 1 g of zinc powder and 20 cm3 of 2 mol dm–3 hydrochloric acid at room temperature.
(b) The rate of reaction of set I is higher than that of set II.
(c) This is because the concentration of hydrochloric acid used in set I is higher than that in set II. - When investigating experimentally the effect of concentration on the rate of reaction,
- the experiment is repeated a few times, each time using a different concentration of a reactant
- all the other factors/conditions are kept constant in all the experiments.
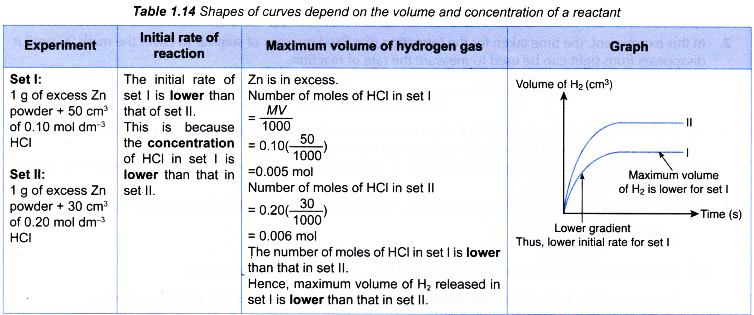
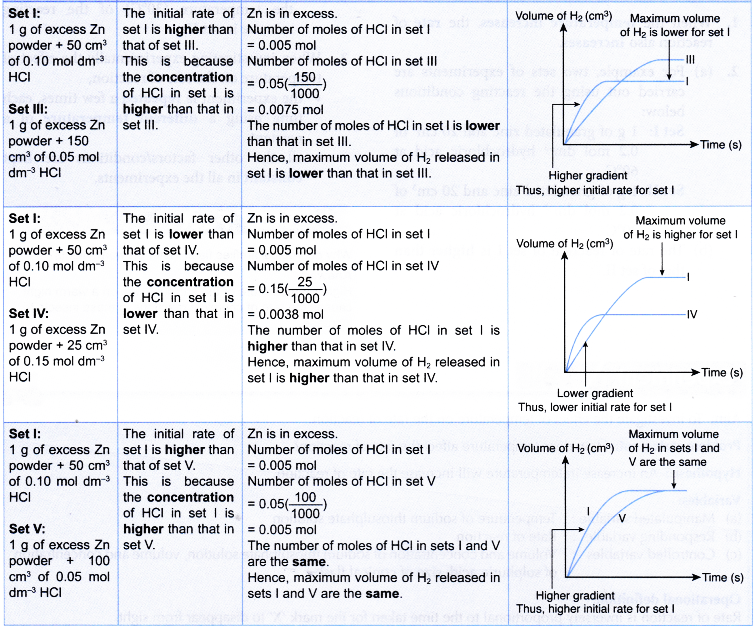
Other suitable reactions to study the effect of concentration on the rate of reaction.
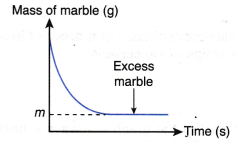
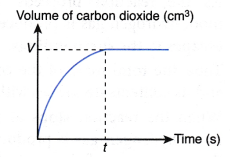
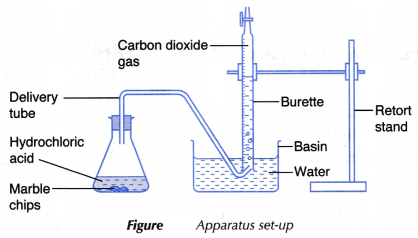
- The effect of concentration of a dilute acid on the rate of reaction involving the liberation of a gas such as the reaction between a reactive metal (Mg/Al/Zn/Fe) and a dilute acid (HCl/H2SO4) to liberate hydrogen gas, a carbonate salt and a dilute acid to liberate carbon dioxide gas can also be investigated experimentally.
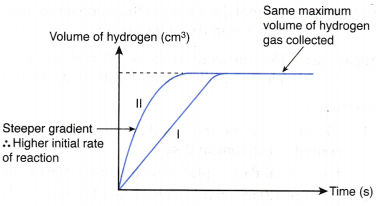
- The experiment is carried out twice by changing the concentration of the dilute acid.
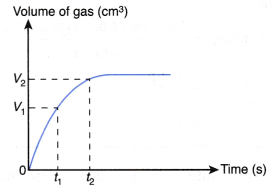
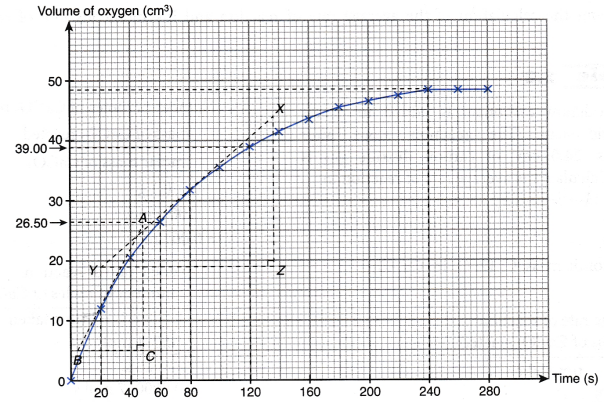
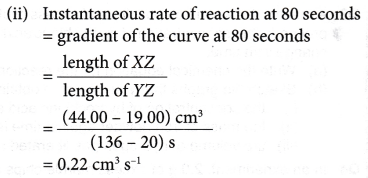
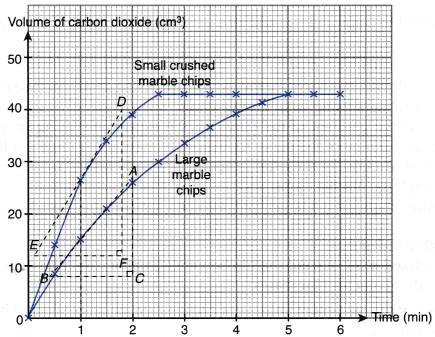
- The curves for the volume of gas liberated against time for both sets of the experiments are plotted on the same axes.
- The gradients of the curves are compared to deduce the difference in rates.
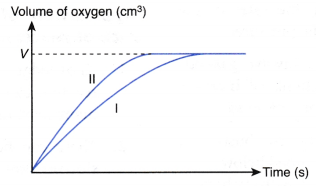
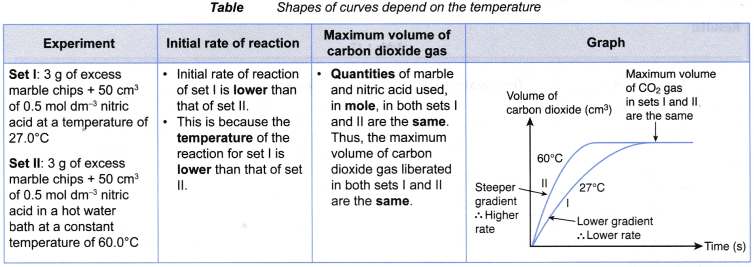
- Different shapes of curves may be obtained depending on the volume and concentration of the dilute acid used.
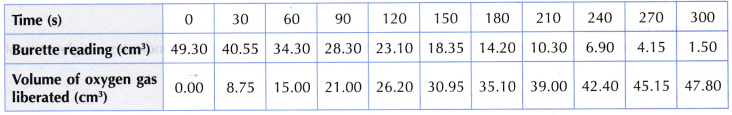
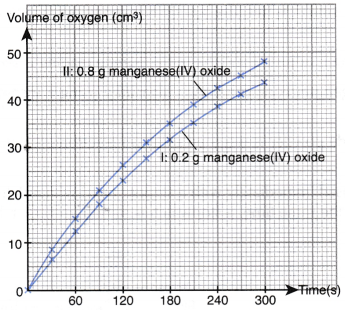
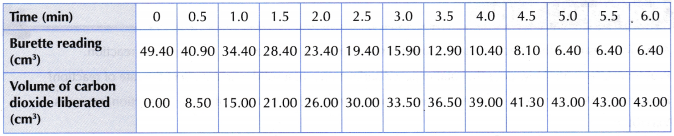
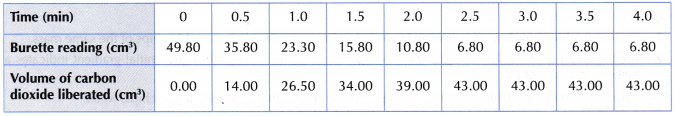
Table illustrates some examples.
People also ask
- What is the rate of the reaction?
- How do you calculate the reaction rate?
- What factors affect the rate of a reaction?
- How does the surface area affect the rate of reaction?
- How does the temperature affect the rate of a chemical reaction?
- What is the effect of a catalyst on the rate of a reaction?
- What is the collision theory in chemistry?
- How does the collision theory affect the rate of reaction?
How does concentration affect the rate of reaction experiment
Aim: To investigate the effect of concentration on the rate of reaction.
Problem statement: How does the concentration of a reactant affect the rate of reaction?
Hypothesis: When the concentration of a reactant increases, the rate of reaction becomes higher.
Variables:
(a) Manipulated variable : Concentration of sodium thiosulphate solution
(b) Responding variable : Rate of reaction
(c) Controlled variables : Temperature, total volume of the reacting mixture, concentration and volume of sulphuric acid, size of conical flask
Operational definition:
Rate of reaction is inversely proportional to the time taken for the mark ‘X’ to disappear from sight.

Materials: 0.2 mol dm–3 sodium thiosulphate solution, 1.0 mol dm–3 sulphuric acid, distilled water, white paper with a mark ‘X’ at the centre.
Apparatus: 150 cm3 conical flasks, 50 cm3 measuring cylinder, 10 cm3 measuring cylinder, digital stopwatch (electronically operated with an accuracy of 0.01 s).
Procedure:
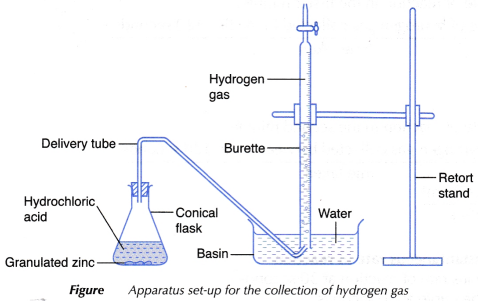
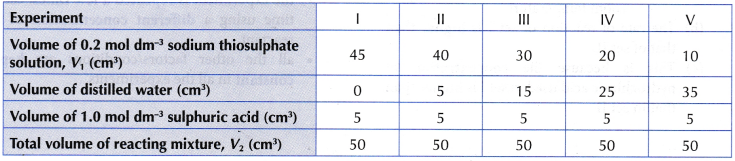
- 45 cm3 of 0.2 mol dm–3 sodium thiosulphate solution is measured using a 50 cm3 measuring cylinder and poured into a conical flask.
- The conical flask is placed on top of a piece of white paper with a mark ‘X’ at the centre.
- 5 cm3 of 1.0 mol dm–3 sulphuric acid is measured using a 10 cm3 measuring cylinder.
- The sulphuric acid is then poured quickly and carefully into the conical flask and a stopwatch is started immediately.
- The mixture in the conical flask is swirled a few times. The conical flask is then placed back on the white paper.
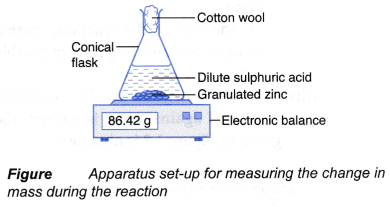
- The mark ‘X’ is viewed vertically from the top through the solution, as shown in Figure.
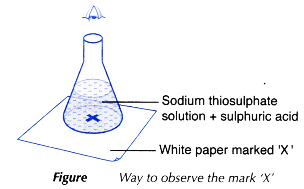
- The stopwatch is stopped immediately once the mark ‘X’ disappears from sight.
- The time t required for the mark ‘X’ to disappear from sight is recorded.
- The experiment is repeated four more times using different volumes of 0.2 mol dm–3 sodium thiosulphate solution diluted with different volumes of distilled water, as shown in Table.
- The results are recorded.
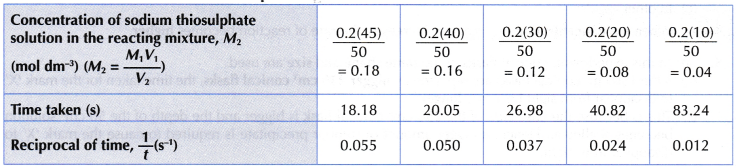
Results:
Interpreting data:
Based on the results obtained, two graphs are plotted.
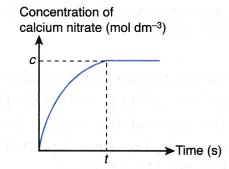
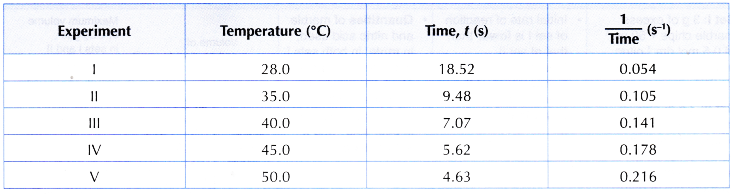
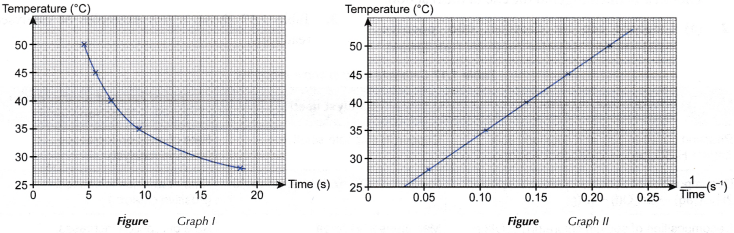
(a) Graph I: Graph of the concentration of sodium thiosulphate solution against time, as shown in figure.
(b) Graph II: Graph of the concentration of sodium thiosulphate solution against 1/time, as shown in figure.

Discussion:
- Sodium thiosulphate solution reacts with dilute sulphuric acid at a very low rate to form a yellow precipitate of sulphur. The chemical equation for the reaction is:
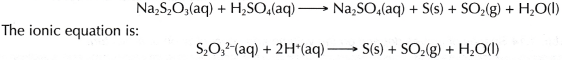
- In this experiment, the time taken for the formation of a fixed quantity of sulphur to cover the mark ‘X’ until it disappears from sight can be used to measure the rate of reaction.
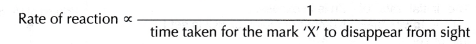
- (a) Based on graph I, it can be seen that as the concentration of sodium thiosulphate solution decreases, a longer time is needed for mark ‘X’ to disappear from sight.
(b) Hence, it infers that as the concentration of sodium thiosulphate solution becomes lower, the rate of reaction also decreases. - (a) Graph II is a straight line. Thus, it implies that the concentration of sodium thiosulphate solution is directly proportional to 1/time.
(b) Since rate of reaction is directly proportional to 1/time, it can be deduced that:
Rate of reaction is directly proportional to the concentration of sodium thiosulphate solution.
(c) In other words:
When the concentration of a reactant increases, the rate of reaction becomes higher. - (a) In this experiment, conical flasks of the same shape and size are used.
(b) If the 150 cm3 conical flasks are replaced by bigger 250 cm3 conical flasks, the time taken for the mark ‘X’ to disappear from sight becomes longer.
(c) This is because the base area of the 250 cm3 conical flask is bigger and the depth of the 50 cm3 solution becomes shallower. Hence, a bigger amount of sulphur precipitate is required to cause the mark ‘X’ to disappear from sight. - (a) in this experiment, if hydrochloric acid of the same concentration is used to replace sulphuric acid, the rate of reaction will become lower.
(b) This is because hydrochloric acid is a strong monoprotic acid, whereas sulphuric acid is a strong diprotic acid. Hence, the concentration of hydrogen ions in hydrochloric acid is only half the concentration of hydrogen ions in sulphuric acid. - Total volume of the reacting mixture in all the five sets of the experiments are the same, that is, 50 cm3. This is to ensure that the quantity of sulphur required for the mark ‘X’ to disappear from sight is the same for all the five sets of the experiments.
Conclusion:
When the concentration of a reactant increases, the rate of reaction also increases. Hence, the hypothesis can be accepted.