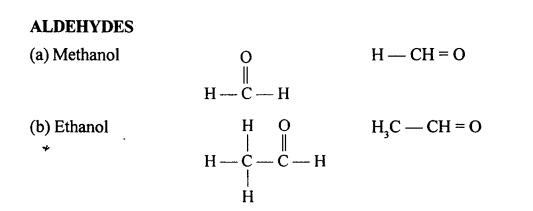
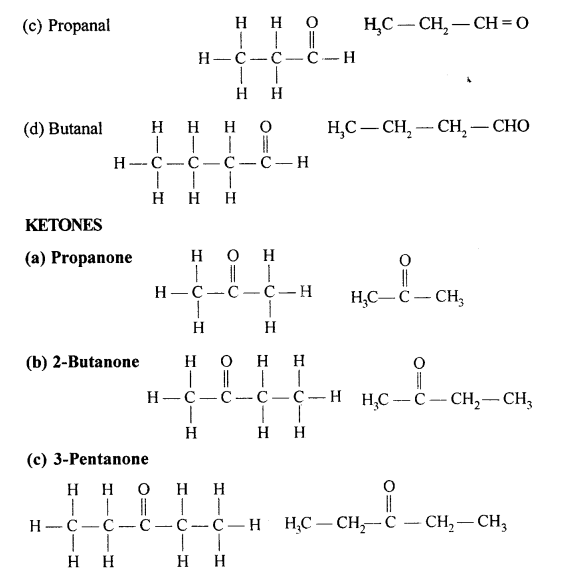
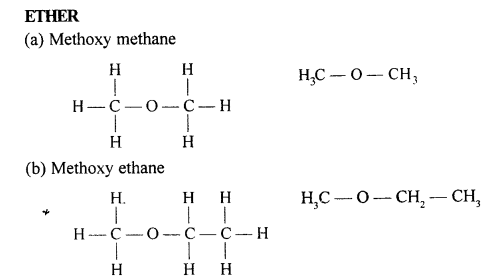
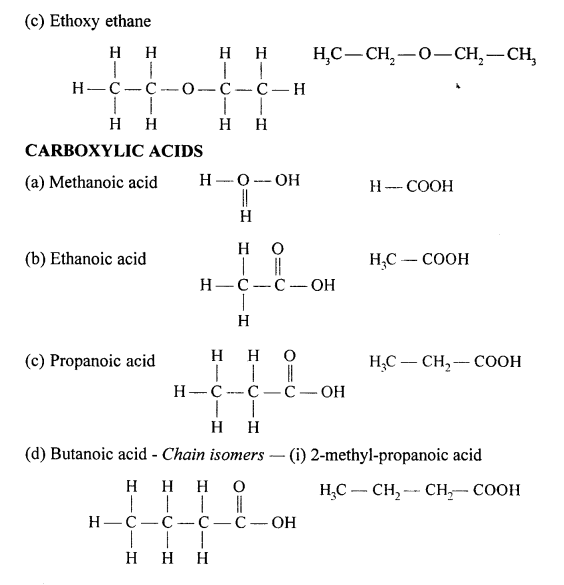
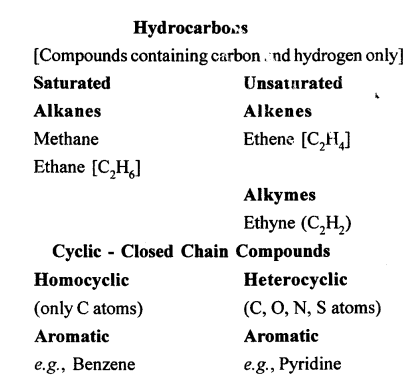
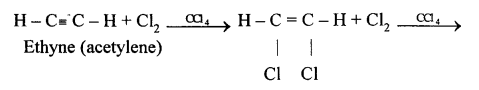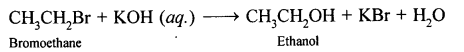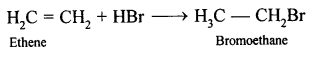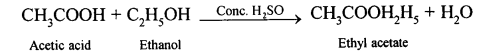Selina Concise Chemistry Class 10 ICSE Solutions Organic Chemistry
APlusTopper.com provides step by step solutions for Selina Concise ICSE Solutions for Class 10 Chemistry Chapter 12 Organic Chemistry. You can download the Selina Concise Chemistry ICSE Solutions for Class 10 with Free PDF download option. Selina Publishers Concise Chemistry for Class 10 ICSE Solutions all questions are solved and explained by expert teachers as per ICSE board guidelines.
Download Formulae Handbook For ICSE Class 9 and 10
ICSE SolutionsSelina ICSE Solutions
Selina ICSE Solutions for Class 10 Chemistry Chapter 12 Organic Chemistry
Exercise 12(A)
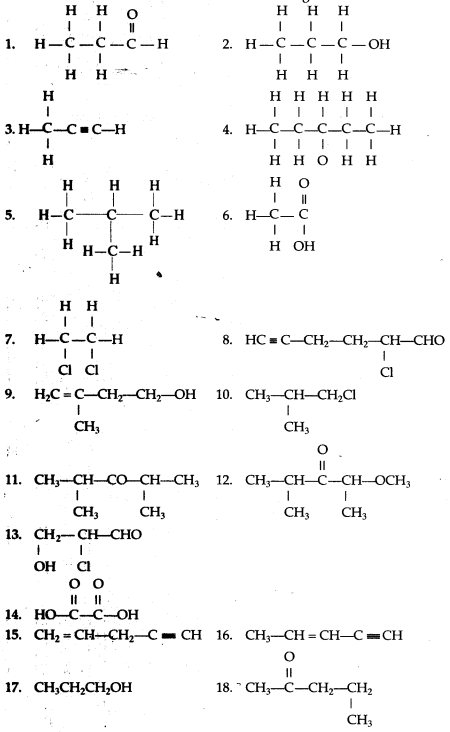
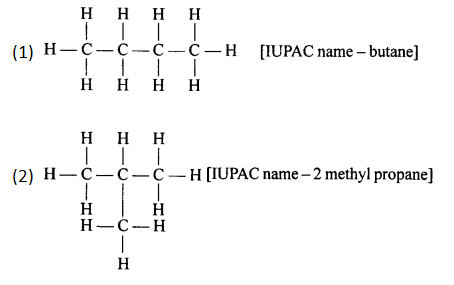
Solution 1.
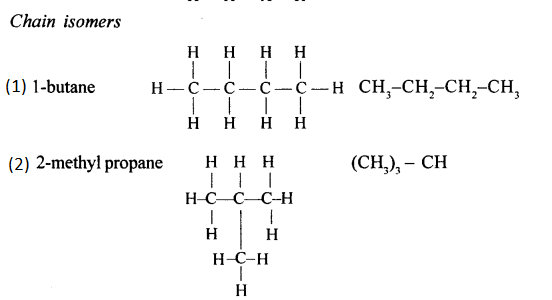
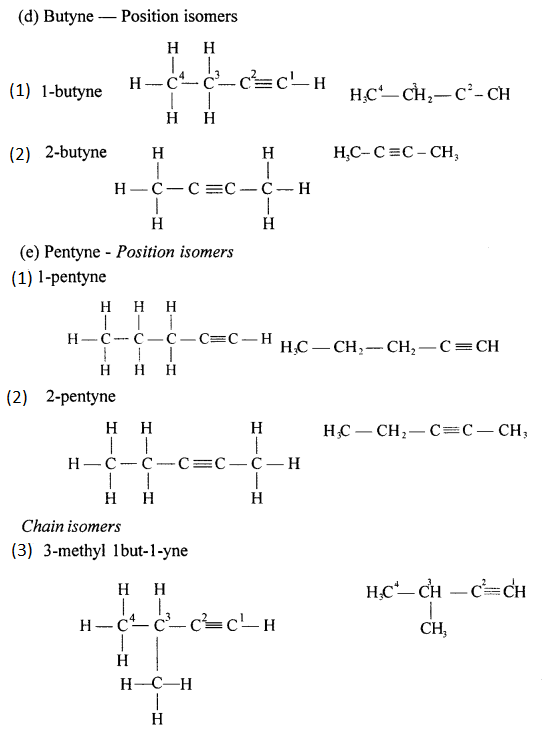
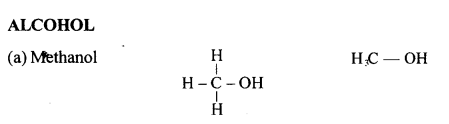
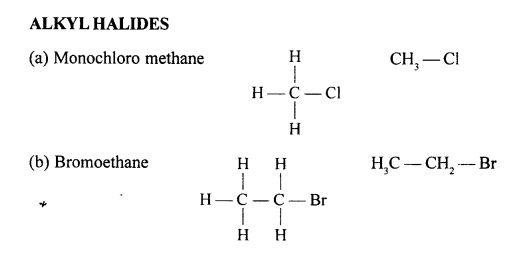
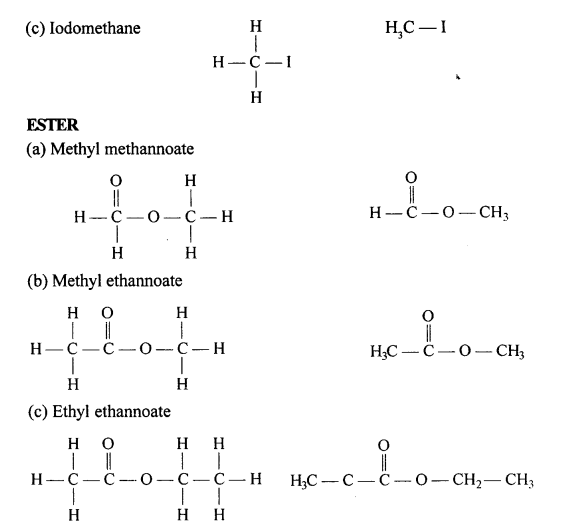
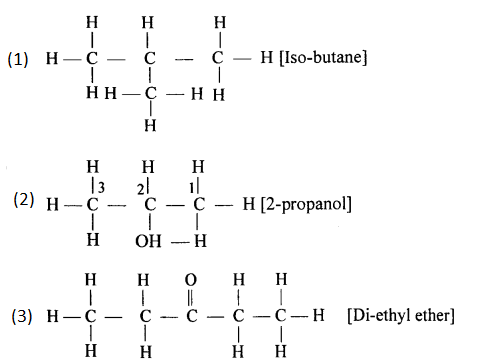
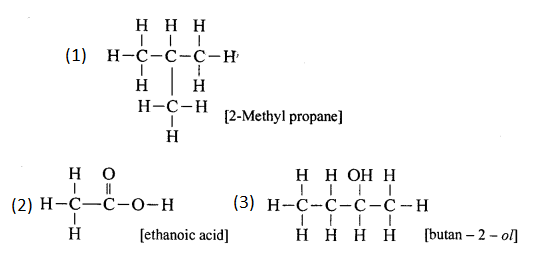
(a) 2,2- dimethylpropane
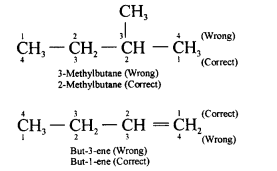
(b) 2-methyl butane
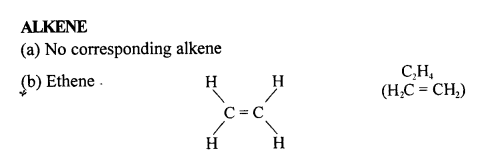
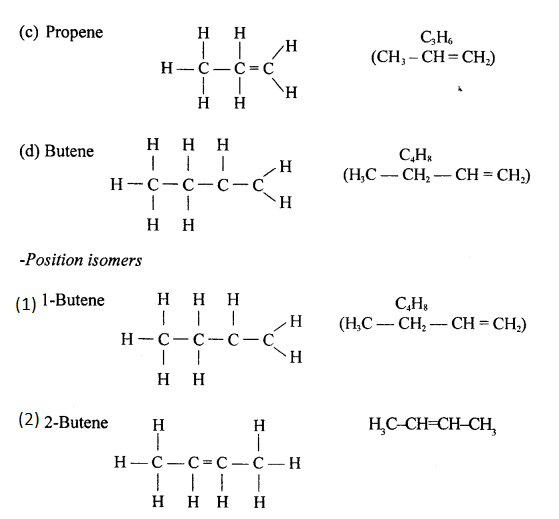
(c) Prop-1-ene
(d) 2,2- dimethyl pentane
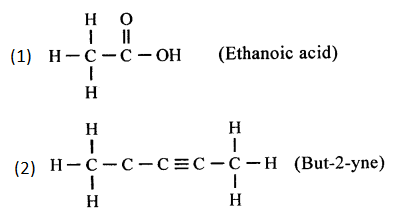
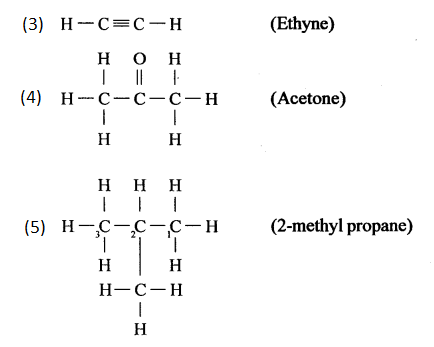
(e) Pent-2-yne
(f) 3-methyl but-1-yne
(g) 2,3-dichloropentane
(h) 3-methylheptane
(i) 2-methyl butane
(j) Hept-2-yne
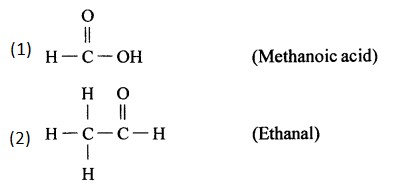
(k) 2,2- dimethyl hexanal
(l) Pentan-2-ol
(m) 4-methylpentanoic acid
(n) 2-bromo2-methyl butane
(o) 1- bromo3-methyl butane
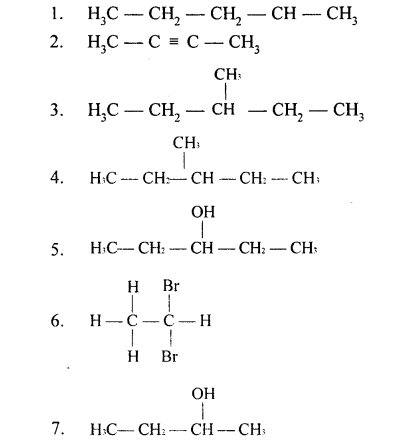
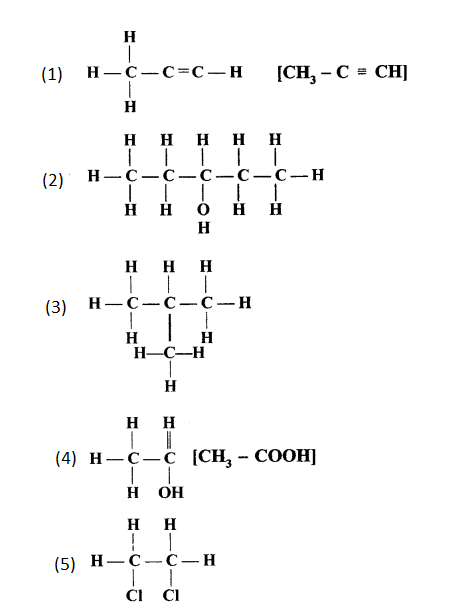
Solution 2.
The structure of the following compounds are:
(a) Prop-1-ene
CH3-CH=CH2
(b) 2,3-dimethylbutane
CH3-CH(CH3)-CH(CH3)-CH3
(c) 2-methylpropane
CH3-CH(CH3)-CH3
(d) 3-hexene
CH3-CH2-CH=CH-CH2-CH3
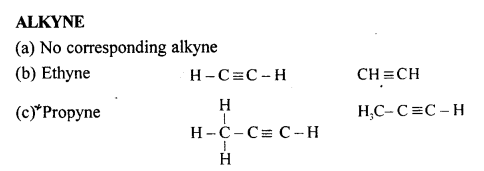
(e) Prop-1-yne
CH3-C≡CH
(f) 2-methylprop-1-ene
CH3-C(CH3)=CH2
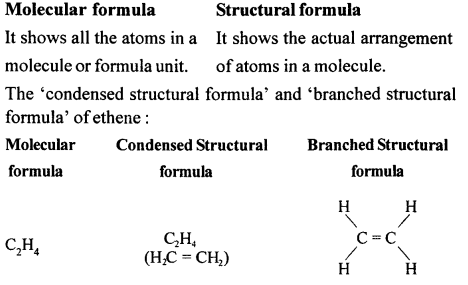
(g) Alcohol with molecular formula C4H10O
CH3-CH2-CH2-CH2-OH
Solution 3.
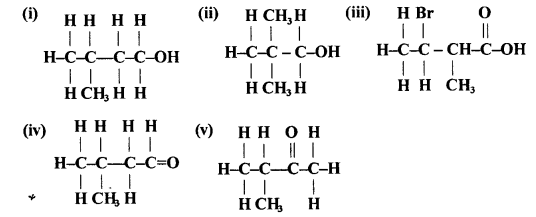
(a) Correct answer: (iv)
CnH2n+1 is the formula for alkyl group. Hence it is C5H11.
(b) Correct answer: (i)
A hydrocarbon of general CnH2n is C15H30.
(c) Correct answer: (ii)
As the formula of Alkene is CnH2n.
Thus n+2n = 72
3n = 72
n = 24
By filling value we get the molecular mass 72.
(d) (iv)
The total number of carbon chains that four carbon atoms form in alkane is 2. They are:

(e) Correct answer: (iv)
Alcohol and ether are functional isomers as they have same molecular formula but different functional groups.
(f) Correct answer: (ii)

Solution 4.
(a) Propane and ethane are homologues.
(b) A saturated hydrocarbon does not participate in a/an addition reaction.
(c) Succeeding members of a homologous series differ by CH2.
(d) As the molecular masses of hydrocarbons increase, their boiling points Increase and melting point increase.
(e) C25H52 and C50H102 belong to the same homologous series.
(f) CO is an organic Compound.
(g) The physical and chemical properties of an organic compound are largely decided by the Functional group.
(h) CHO is the functional group of an aldehyde.
(i) The root in the IUPAC name of an organic compound depends upon the number of carbon atoms in Principal Chain.
(j) But-1-ene and but-2-ene are examples of position isomerism.
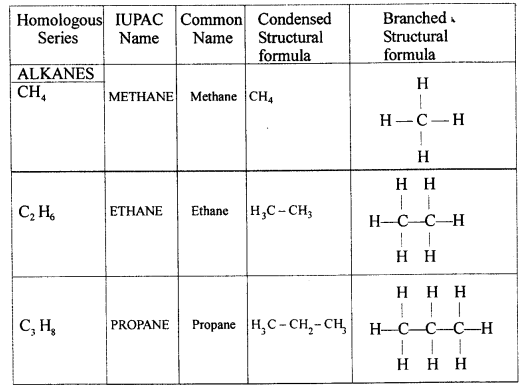
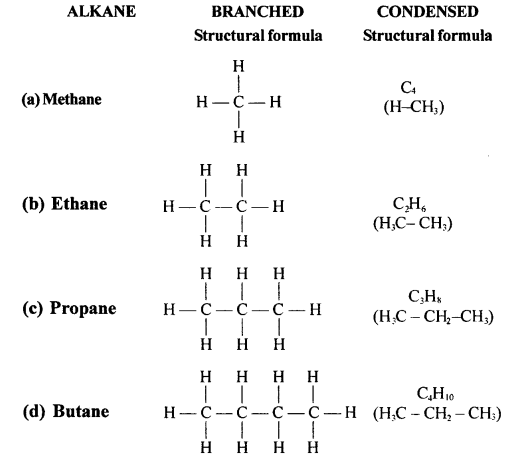
Exercise 12(B)
Solution 1.
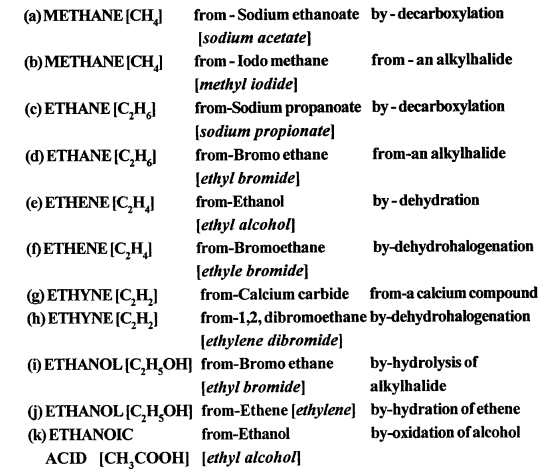
Sources of alkane:
The principal sources of alkanes are Natural gas and petroleum.
Solution 2.
Methane is a primary constituent of natural gas. It absorbs outgoing heat radiation from the earth, and thus contributes to the green house effect and so it is considered as a green house gas.
Solution 3.
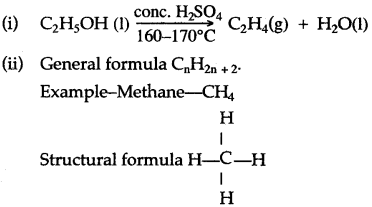
The general formula of alkane is :
CnH 2n+2
Solution 4.

Solution 5.

Solution 6.
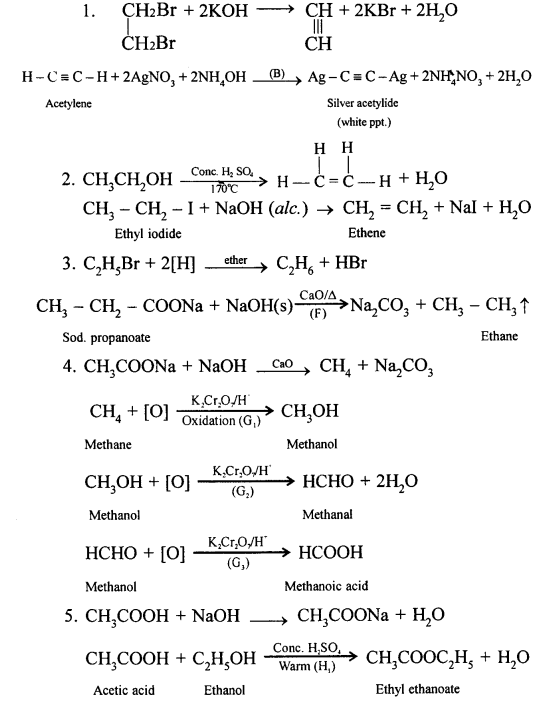
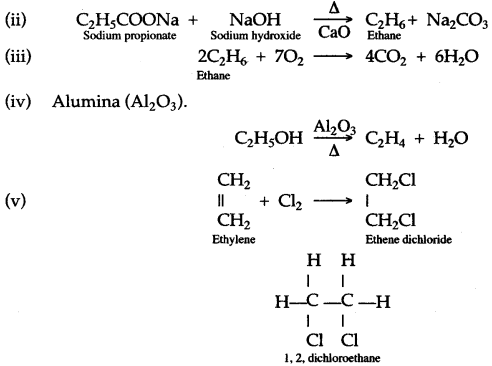
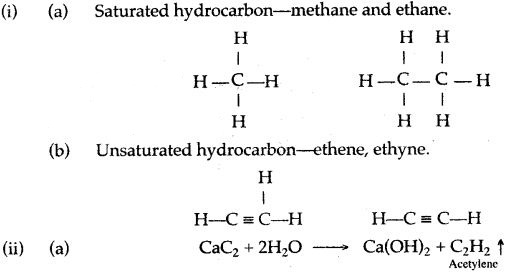
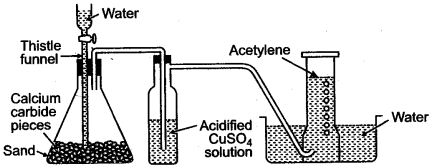
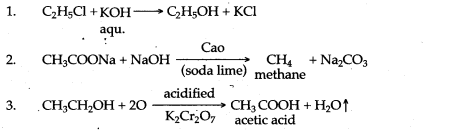
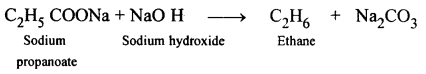
(a) Laboratory preparation of methane:
When the mixture of sodium ethanoate and soda lime is taken in a hard glass test tube and heated, the gas evolved is methane. It is collected by downward displacement of water.
![]()
(b) Laboratory preparation of ethane:
When the mixture of sodium propionate and soda lime is taken in the boiling tube and heated the ethane gas is evolved. It is also collected by downward displacement of water.
![]()
Solution 7.
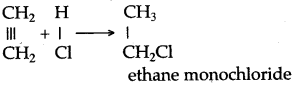
When methyl iodide is reduced by nascent hydrogen at ordinary room temperature then methane is formed.
CH3I + 2[H] → CH4 + HI
When bromoethane is reduced by nascent hydrogen at ordinary room temperature then ethane is produced.
C2H5Br + 2[H] → C2H6 + HBr
Solution 8.

Solution 9.
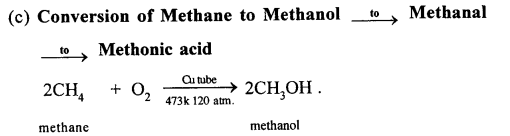
(a) Sufficient air: When methane burns in sufficient air, then carbon dioxide and water vapors are formed.
CH4 + 2O2 → CO2+2H2O
(b) Insufficient air: When methane burns in insufficient air , then carbon monoxide and water is formed.
2CH4 + 3O2 → 2CO + 4H2O
Solution 10.
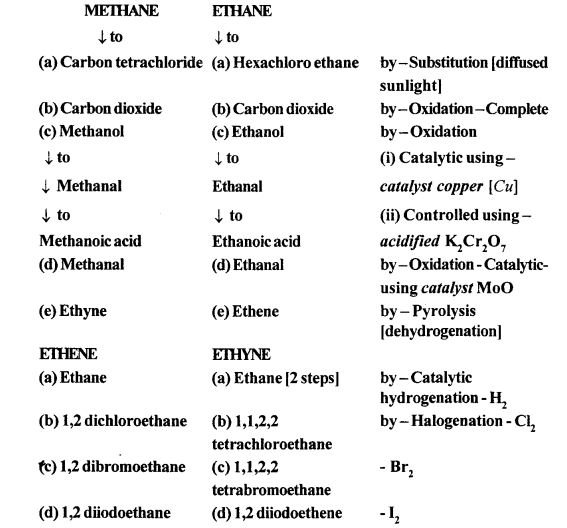
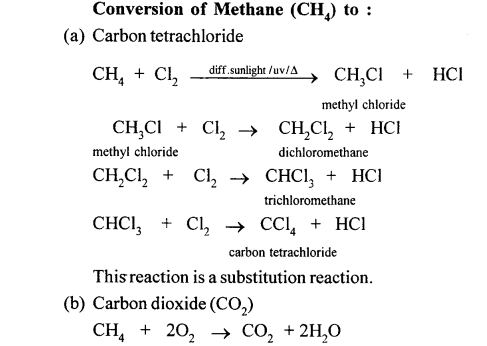
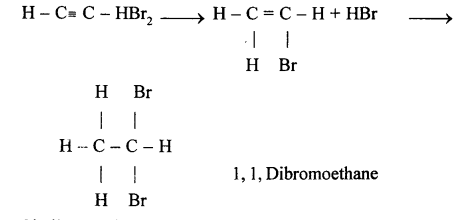
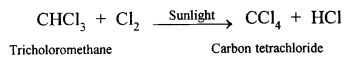
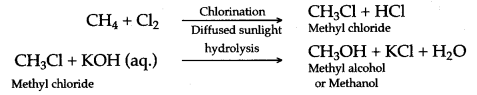
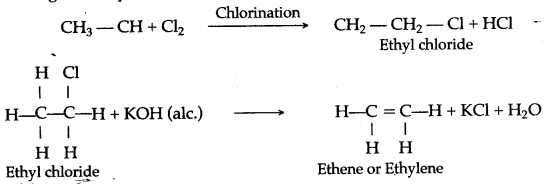
(a) (i) When methane reacts with chlorine in the presence of sunlight or UV light, it undergoes substitution reaction to form Tetrachloromethane.

(ii) When it reacts with bromine it forms Tetrabromomethane
CH4 + Br2 → CH3Br + HCl
CH3Br + Br2 → CH2Br2 + HCl
Dibromomethane
CH2Br2 + Br2 → CHBr3 + HCl
Tribromo methane
CHBr3 + Br2 → CBr4 + HCl
Tetrabromomethane
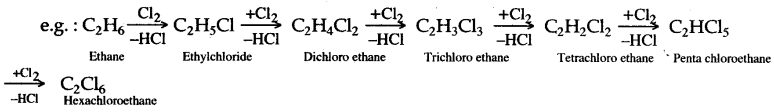
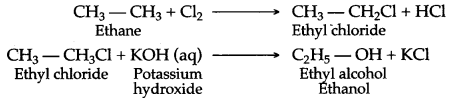
(b) (i) When ethane reacts with chlorine it forms hexachoroethane.
C2H6 + Cl2 → C2H5Cl + HCl
Chloroethane
C2H5Cl + Cl2 → C2H4Cl2 + HCl
Dichloroethane
C2H4Cl2 + Cl2 → C2H3Cl3+ HCl
Trichloroethane
C2H3Cl3 + Cl2 → C2H2Cl4 + HCl
Tetrachloroethane
C2H2Cl4 +Cl2 → C2HCl5 + HCl
Pentachloroethane
C2HCl5 + Cl2 → C2Cl6 + HCl
Hexachloroethane
(ii) When ethane reacts with bromine it forms Hexabromoethane
C2H6 +Br2 → C2H5Br + HBr
Bromoethane
C2H5Br + Br2 → C2H4Br2+HBr
Dibromoethane
C2H4Br2 +Br2 → C2H3Br3+HBr
Tribromoethane
C2H3Br3 + Br2 → C2H2Br4 + HBr
Tetrabromoethane
C2H2Br4 +Br2 → C2HBr5 +HBr
Pentabromoethane
C2HBr5 +Br2 → C2Br6 + HBr
HexaBromoethane
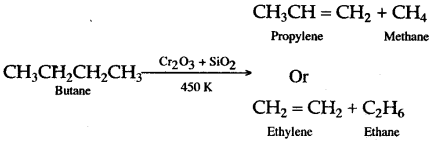
Solution 11.

Solution 12.
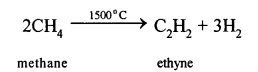
The decomposition of a compound by heat in the absence of air is called Pyrolysis. When pyrolysis occurs in alkanes, the process is termed cracking.
For example:
Alkanes on heating under high temperature or in the presence of a catalyst in absence of air broken down into lower alkanes, alkenes and hydrogen.
![]()
Solution 13.

Solution 14.
(a) Methane: Three uses of methane are:
- Methane is a source of carbon monoxide and hydrogen
- It is used in the preparation of ethyne, methanal, chloromethane, carbon tetrachloride.
- It is employed as a domestic fuel.
(b) Ethane: Three uses of ethane are:
- It is used in the preparation of ethene, ethanol, and ethanol.
- It forms ethyl chloride, which is used to make tetraethyllead.
- It is also a good fuel.
Solution 15.
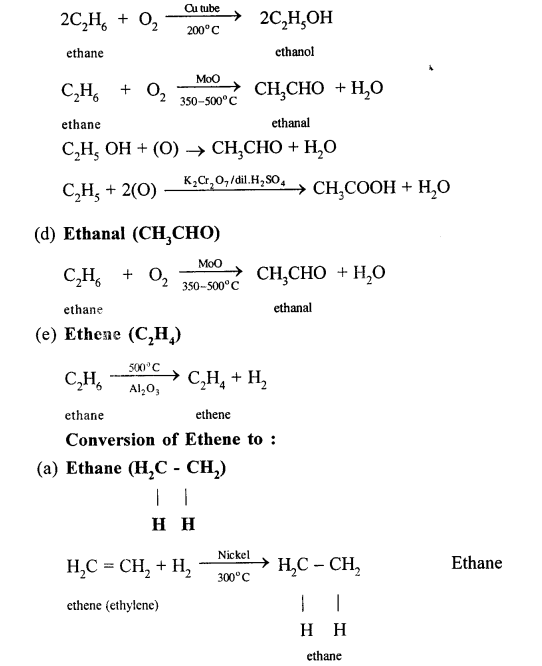
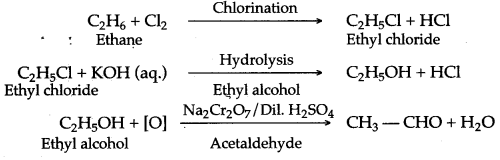
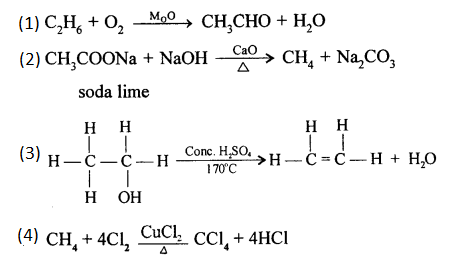
(a) When a mixture of ethane and oxygen is compressed to about 120atm pressure and passed over copper tubes at 475K, ethyl alcohol is formed.
![]()
(b) When mixture of ethane and oxygen is passed through heated molybdenum oxide, the mixture is oxidized to Acetaldehyde.

Solution 16.
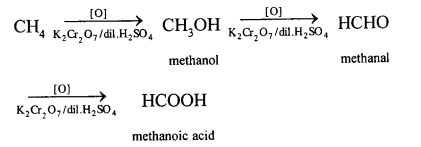
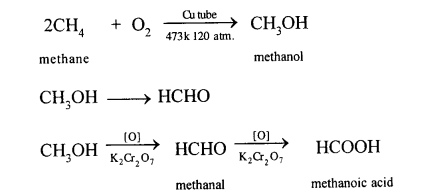
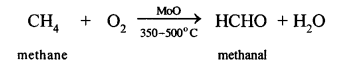
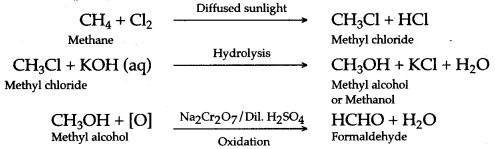
(a) Methane to methyl alcohol:
When a mixture of methane and oxygen is compressed to about 120atm pressure and passed over copper tubes at 475K, ethyl alcohol is formed.
![]()
(b) Methane to formaldehyde:
When mixture of methane and oxygen is passed through heated molybdenum oxide, the mixture is oxidized to Formaldehyde

Exercise 12(C)
Solution 1.

Solution 2.
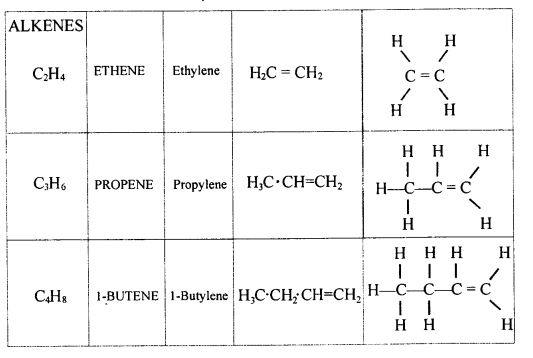
(a) n signifies the number of carbon atoms and 2n signifies the number of hydrogen atoms.
(b) The name of alkene when n=4 is Butene.
(c) The molecular formula of alkene when n=4 is C4H8.
(d) The molecular formula of alkene when there are 10 H atom in it C5H10.
(e) The structural formula of the third member of alkene is

(f) Lower homologus of alkene which contain four carbons is C3H6.
Higher homologus of alkene which contain four carbons is C5H10.
Solution 3.
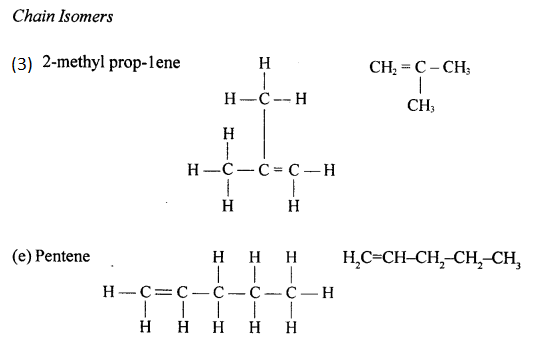
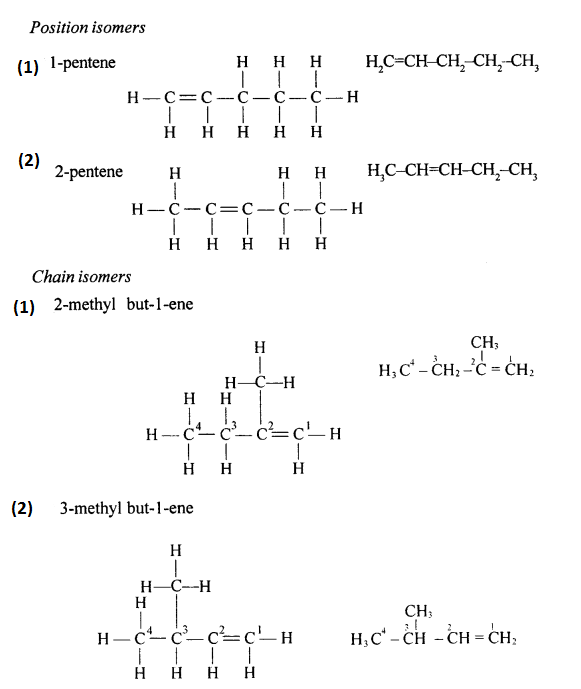
The isomers of Butene are:
(i) CH3-CH2-CH=CH2 , But-1-ene
(ii) CH3-CH=CH-CH3 , But-2-ene
(iii) CH2=C(CH3)-CH3 , 2-methyl propene
Solution 4.

Solution 5.

Solution 6.
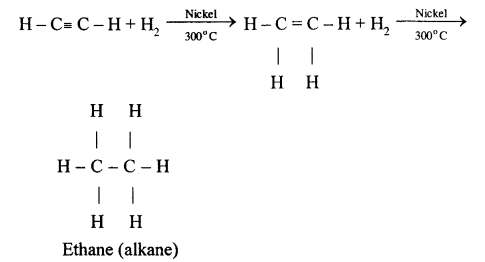
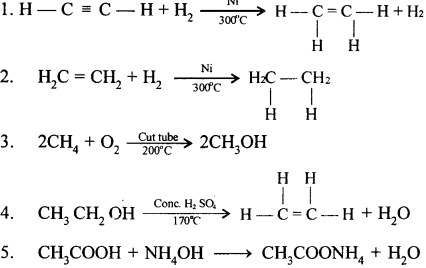
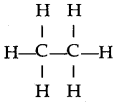
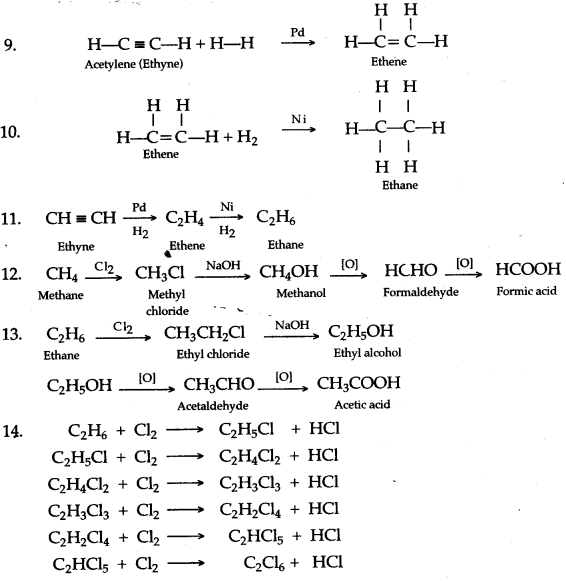
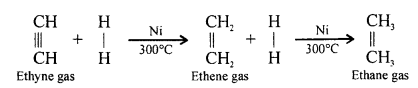
When ethene and hydrogen are passed over finely divided catalyst such as platinum or palladium at ordinary temperature or nickel at 200o C, the two atom of hydrogen molecule are added to the unsaturated molecule, which thus becomes a saturated one.
![]()
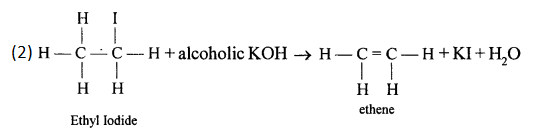
Solution 7.
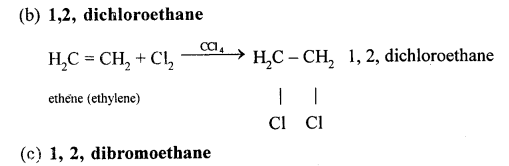
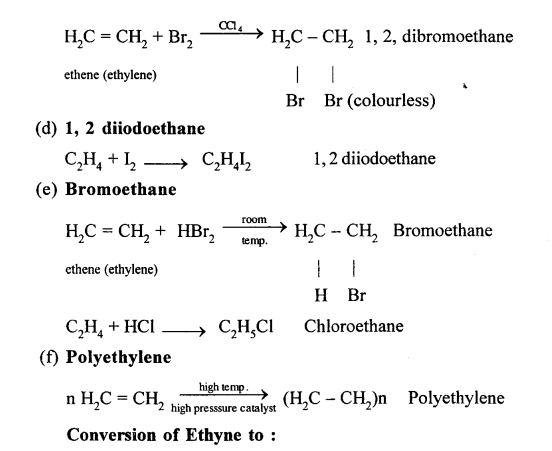
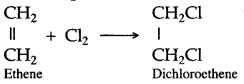
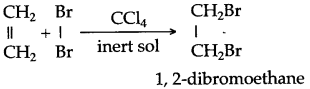
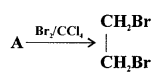
Chlorine and bromine are added to the double bond of ethene to form saturated ethylene chloride and ethylene bromide respectively.
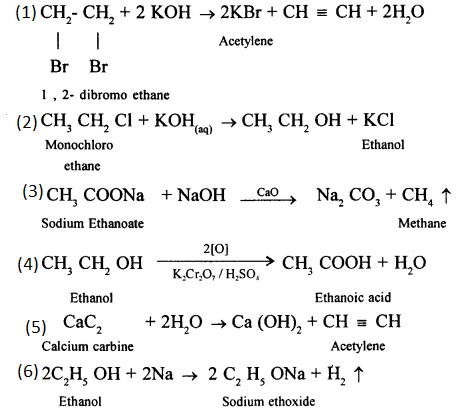
CH2 = CH2 + Cl2 → CH2(Cl)-CH2(Cl)
1,2-dichloro ethane
CH2 = CH2 + Br2 → CH2(Br)-CH2(Br)
1,2-dibromo ethane
Solution 8.

Solution 9.
(a) Physical state: Ethene is a colourless and inflammable gas.
(b) Odour: It has faint sweetish odour.
(c) Density as compared to air: It has density less than one hence it is lighter than air.
(d) Solubility: It is sparingly soluble in water but highly soluble in organic solvents like alcohol, ether and chloroform.
Solution 10.
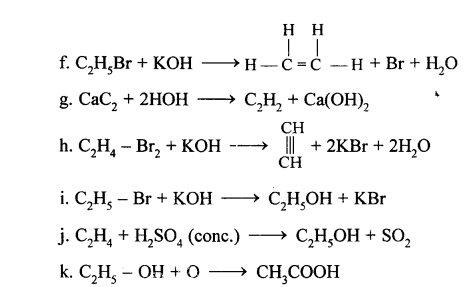
(a) Ethene into 1, 2 -dibromoethane: Ethene reacts with bromine at room temperature to form saturated ethylene chloride.
CH2=CH2 + Br2 → CH2(Br)-CH2(Br)
1,2-dibromo ethane
(b) Ethene into ethyl bromide: When ethene is treated with HBr bromoethane is formed.
CH2=CH2 + HBr → CH3-CH2Br
Ethyl bromide
Solution 11.

Solution 12.

Solution 13.

Solution 14.
When ethylene is passed through alkaline KMnO4 solution 1, 2-Ethanediol is formed. The Purple color of KMnO4 decolorizes.
CH2=CH2+H-O-H + [O] → CH2(OH)-CH2(OH)
Cold alkaline
KMnO4 solution
Solution 15.
Three compounds formed by ethylene are:
- Polythene
- Ethanol
- Epoxyethane
Uses of above compounds:
- Polythene is used as carry bags.
- Ethanol is used as a starting material for other products, mainly cosmetics and toiletry preparation.
- Epoxyethane is used in the manufacture of detergents.
Exercise 12(D)
Solution 1.
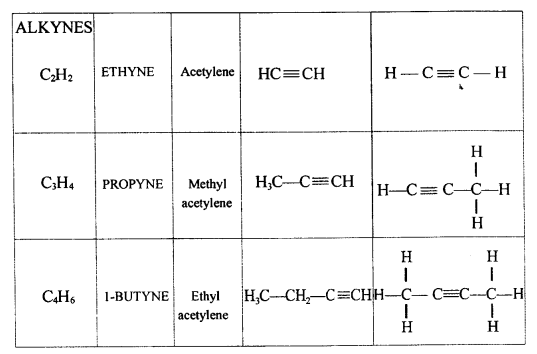
Natural gas and Petroleum are sources for alkynes.
The general formula of alkynes are:
CnH2n-2
Solution 2.

Solution 3.

Solution 4.
![]()
Solution 5.
The following compounds can be classified as:
C3H4:- Alkynes
C3H8:- Alkanes
C5H8:- Alkynes
C3H6:- Alkenes
Solution 6.

Solution 7.
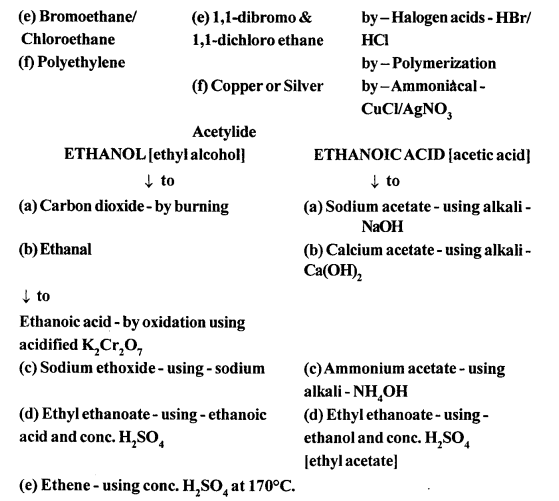
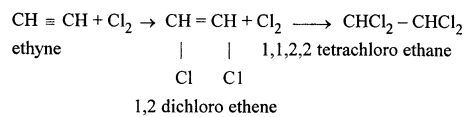
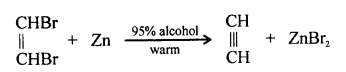
(a) Ethyne in an inert solvent of carbon tetrachloride adds chlorine to change into 1,2-dichloro ethene with carbon-carbon double bond, and then to an 1,1,2,2-tetrachloro ethane with carbon-carbon single bond.![]()
1,2-dichloro ethene 1,1,2,2 -tetrachloro ethane
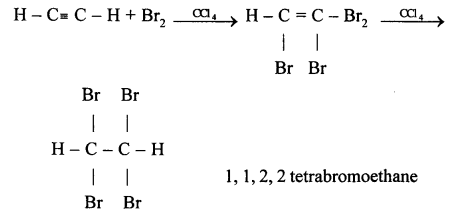
(b) Ethyne in an inert solvent of carbon tetrachloride adds bromine to change into 1,2-dibromo ethene and then to 1,1,2,2 -tetrabromo ethane.

Exercise 12(E)
Solution 1.
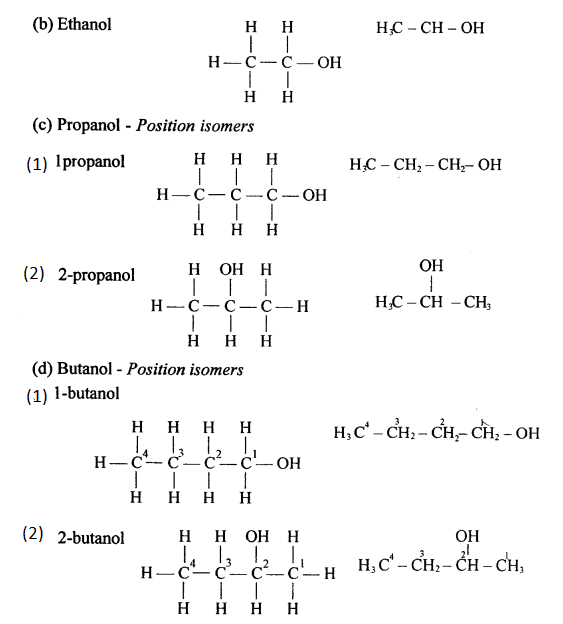
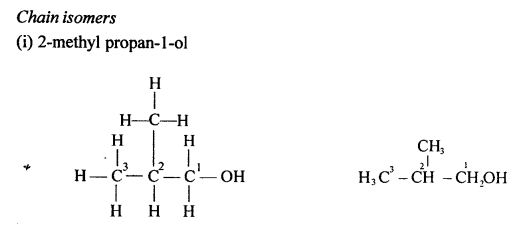
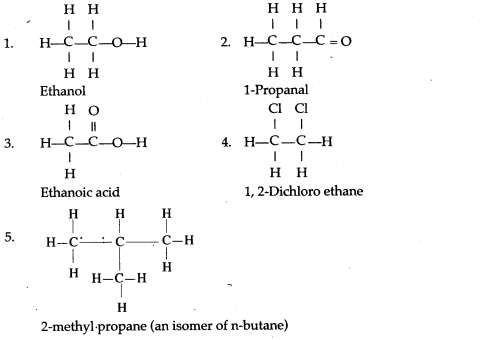
(a) Alcohols are the hydroxyl derivatives of alkanes and are formed by replacing one or more hydrogen atoms of the alkane with OH group.
Methanol is obtained from destructive distillation of wood while ethanol is obtained from fermentation of sugar.
(b) General formula of monohydric alcohol:
CnH2n+ 1OH
Solution 2.

Solution 3.
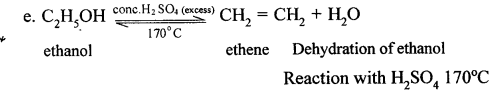
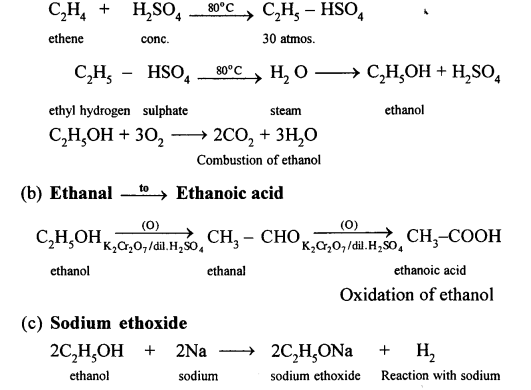
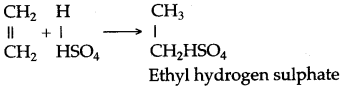
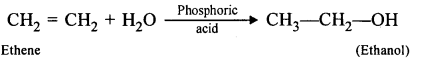
(a) By hydrolysis of ethene: When concentrated sulphuric acid is added to ethene at a temperature of 80oC and pressure of 30 atm. ethyl hydrogen sulphate is produced. Ethyl hydrogen sulphate on hydrolysis with boiling water gives ethanol.

(b) By hydrolysis of alkyl halide: Alcohols can be prepared by the hydrolysis of alkyl halide with a hot dilute alkali.
![]()
Solution 4.

Solution 5.

Solution 6.
(a) The melting and boiling point of the successive members of the homologous series of alcohols increase with the increase in molecular mass.
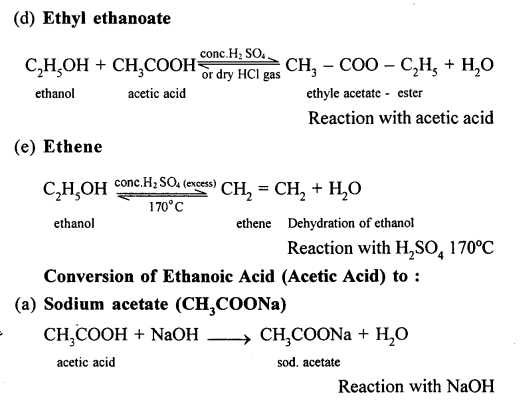
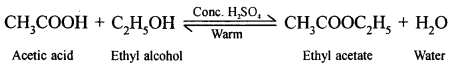
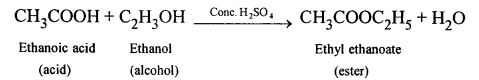
(b) When ethanol reacts with acetic acid ethyl acetate is formed.
![]()
Solution 7.
Ethanol affects that part of the brain which controls our muscular movements and then gives temporary relief from tiredness. But it damages the liver and kidney too.
Solution 8.
(a) Absolute alcohol: Absolute alcohol may be obtained by distilling moist alcohol with benzene. The mixture of water and benzene distills off and anhydrous alcohol is left behind.
(b) Spurious alcohol: It is made by improper distillation. It contains large portions of methanol in a mixture of alcohols.
(c) Methylated spirit: Methylated spirit or denatured alcohol is ethyl alcohol with 5%methyl alcohol, a coloured dye and some pyridine.
Solution 9.

Solution 10.
| S No | Formula | Common Name | IUPAC |
| 1 | C3H6 | Propylene | Propene |
| 2 | C2H4 | Ethylene | Ethene |
| 3 | C2H2 | Acetylene | Ethyne |
| 4 | CH3OH | Methyl alcohol | Methanol |
| 5 | C2H5OH | Ethyl alcohol | Ethanol |
Solution 11.

Solution 12.

Solution 13.
(a) Used for illuminating country houses : Ethyne
(b) Used for making a household plastic material: ethyne
(c) Called ‘wood spirit’ : Methanol
(d) Poisonous: Methanol
(e) Consumed as a drink: Ethanol
(f) Made from water gas: Methanol
Exercise 12(F)
Solution 1.
An organic compound containing the carboxyl group(COOH) is known as carboxylic acid.
The general formula: CnH2n+1COOH
Solution 2.
Monocarboxylic acid:
Formula: HCOOH
Common name: Formic acid
IUPAC name: Methanoic acid
Dicarboxylic acid:
Formula: COOH-COOH
Common name : Oxalic acid
IUPAC name: Ethane-di-oic acid
Solution 3.
(a) First three members of carboxylic acids are:
- Methanoic acid
- Ethanoic acid
- Propanoic acid
(b) Three compounds that can be oxidized directly or in stages to produce acetic acid are:
- Ethanol
- Acetylene
- Ethanal
Solution 4.
Vinegar commonly called Sirka is a dilute solution of acetic acid. The presence of colouring matter gives it a greyish colour while the presence of some other organic acids and organic compounds impart it the usual taste and flavour.
Solution 5.

IUPAC name of acetic acid is: Ethanoic acid
Glacial acetic acid is the pure form of acetic acid. It does not contain water.
Solution 6.
(a) Ethanol
(b) Acetic acid
(c) Propanoic acid
Solution 7.
(a) It is prepared in the lab by the oxidation of ethanol with acidified potassium dichromate.![]()
(b) Acetylene is first converted to acetaldehyde by passing through 40% H2SO4 at 60°C in the presence of 1% HgSO4.
The acetaldehyde is then oxidised to acetic acid in the presence of catalyst manganous acetate at 70°C.
Solution 8.

Solution 9.

Solution 10.
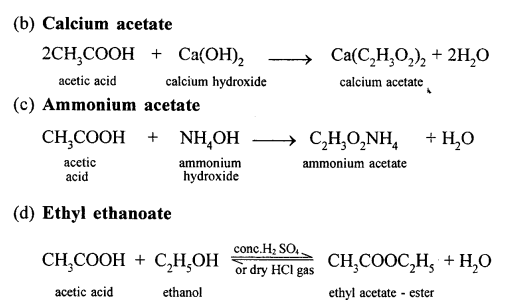
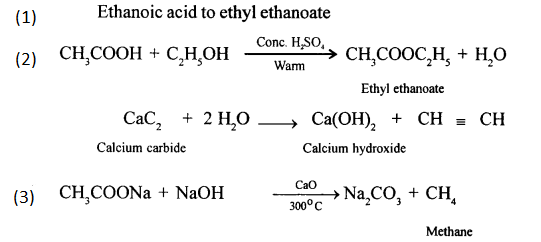
(a) When acetic acid and ethanol react it results in the formation of ethyl acetate.
(b) Lithum aluminium hydride(LiAlH4) is used to convert acetic acid to ethanol.
(c) Phosphorous pentoxide(P2O5) is heated along with acetic acid to form acetic anhydride.
Solution 11.
Test to show that CH3COOH is acidic are:
When litmus test is done, it turns blue litmus red.
It react with bases to form salt and water.
Solution 12.

Exercise Intext 1
Solution 1.
(a) Organic chemistry may be defined as the chemistry of hydrocarbons and its derivatives.
(b) Vital Force Theory is a theory made by the Scientist Berzelius in 1809 which assumed that organic compounds are only formed in living cells and it is impossible to prepare them in laboratories.
It was discarded because Friedrich Wohler showed that it was possible to obtain an organic compound(urea) in the laboratory.
Solution 2.
(a) Few sources of organic compounds are:
Plants, Animals, Coal, Petroleum and Wood.
(b) The various applications of organic chemistry is:
- It is used in the production of soaps, shampoos, powders and perfumes.
- Various fuels like natural gas, petroleum are also organic compounds.
- The fabrics that we use to make various dresses are also made from organic compounds.
Solution 3.
Organic compounds are present everywhere. They are present in:
- It is present in the production of soaps, shampoos, powders and perfumes.
- It is present in the food we eat like carbohydrates, proteins, fats, vitamins etc.
- Fuel like natural gas, petroleum are also organic compounds.
- Medicines, explosives, dyes, insecticides are all organic compounds.
Thus we can say that organic compounds play a key role in all walks of life.
Solution 4.
The unique properties shown by carbon are:
- Tetravalency of carbon
- Catenation
- Isomerism
Solution 5.
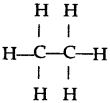
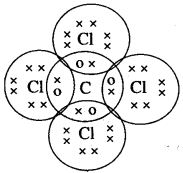
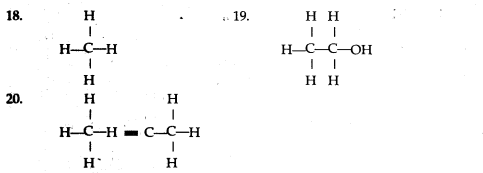
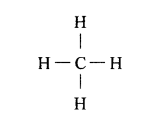
(a) Tetravalency: Carbon can neither lose nor gain electrons to attain octet. Thus it shares four electrons with other atoms. This characteristics of carbon by virtue of which it forms four covalent bonds, is called Tetravalency of carbon.
In structural form :
![]()
(b) Catenation: The property of self -linking of atoms of an element through covalent bonds in order to form straight chains, branched chains and cyclic chains of different sizes is known as catenation.
Carbon- carbon bond is strong so carbon can combine with other carbon atoms to form chains or rings and can involve single, double and triple bonds.
![]()
Solution 6.
Four properties of organic compound that distinguish them from inorganic compounds are:
- Presence of carbon.
- Solubility in the organic solvents.
- Forming of covalent bonds.
- Having low melting and boiling points.
Solution 7.
Due to the unique nature of carbon atom, it gives rise to formation of large number of compounds. Thus this demands a separate branch of chemistry.
Solution 8.
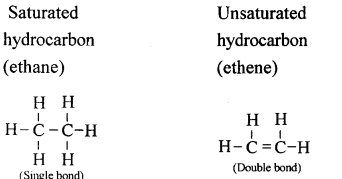
Hydrocarbons are compounds that are made up of only carbon and hydrogen.
Comparison of saturated and Unsaturated hydrocarbons:
| Saturated Hydrocarbon | Unsaturated Hydrocarbon |
| 1. Carbon atoms are joined only by single bonds. | Carbon atoms are joined by double or by triple bonds. |
| 2. They are less reactive due to the non-availability of electrons in the single covalent bond. | They are more reactive due to presence of electrons in the double or the triple bond. |
| 3. They undergo substitution reaction. | They undergo addition reaction. |
Solution 9.
Due to presence of unique properties of carbon like Tetravalency, catenation and Isomerism large number of organic compounds are formed.
Solution 10.

Solution 11.

Solution 12.
The member of each of the following is:
(a) Saturated Hydrocarbon: Hexane (C6H14)
(b) Unsaturated Hydrocarbon: Hexene (C6H12)
Solution 13.
Substitution reaction: A reaction in which one atom of a molecule is replaced by another atom (or group of atoms) is called a substitution reaction.
Addition reaction: A reaction involving addition of atom(s) or molecules(s) to the double or the triple bond of an unsaturated compound so as to yield a saturated product is known as addition reaction.
Solution 14.
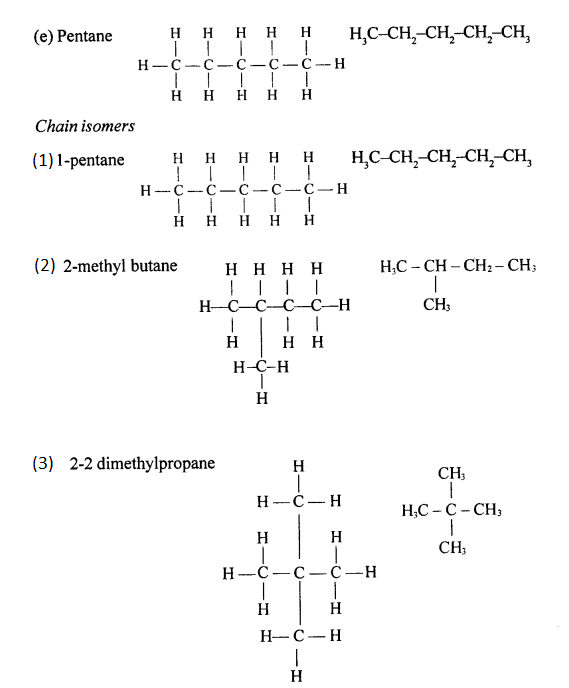
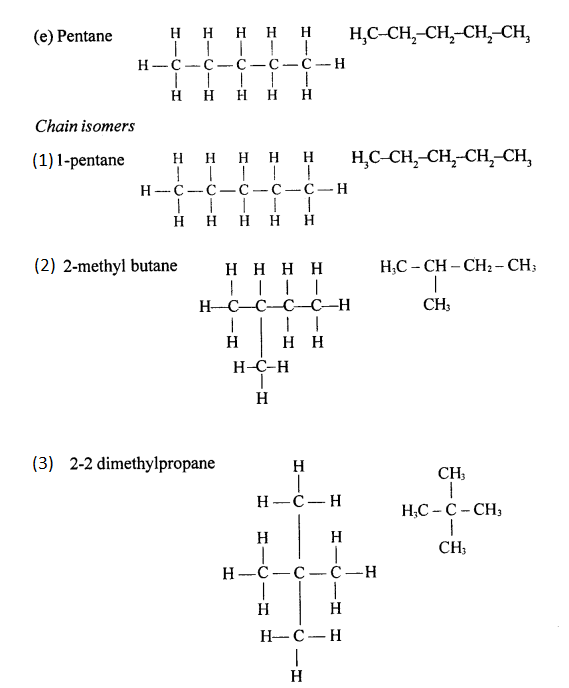
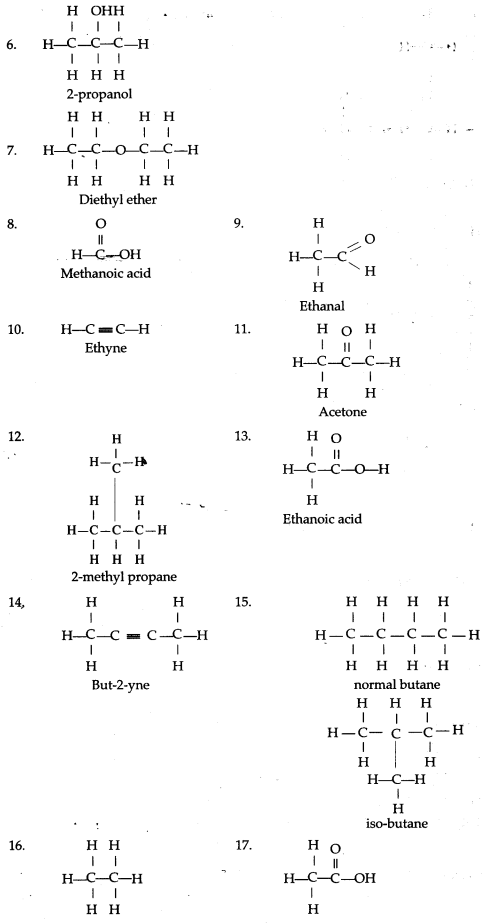
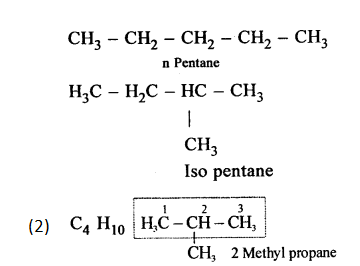
Chain isomerism
Chain isomerism arises due to the difference in arrangement of C atoms in the chain. For example, there are two isomers of butane, C4H10. In one of them, the carbon atoms lie in a “straight chain” whereas in the other the chain is branched.

Position isomerism
It is due to the difference in position of functional groups.
For example, there are two structural isomers with the molecular formula C3H7Br. In one of them, the bromine atom is on the end of the chain, whereas in the other it is attached in the middle.

Solution 15.
(a) Isomerism: Compounds having the same molecular formula but different structural formula are known as isomers and the phenomenon as isomerism.
Two main causes of isomerism are:
Difference in mode of linking of atoms.
Difference in the arrangement of atoms or groups in space.

Solution 16.
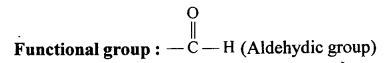
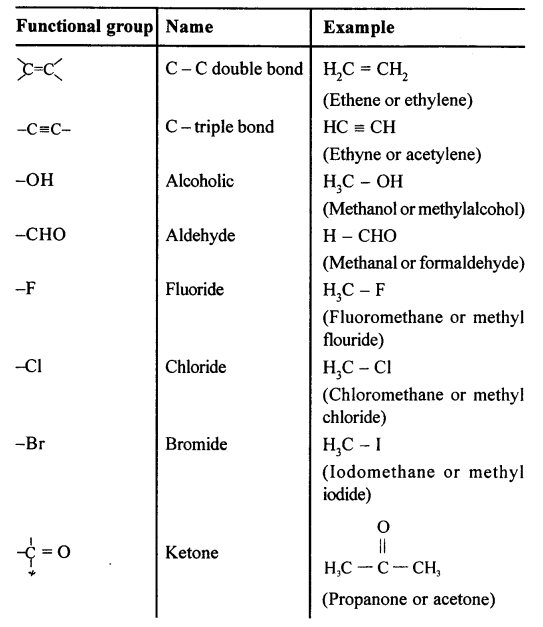
A functional group is an atom or a group of atoms that defines the structure (or the properties of a particular family) of organic compounds.
The structural formula of

Solution 17.
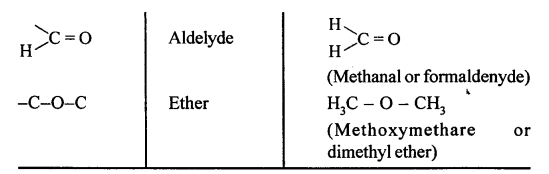
The functional group present in the following compounds are:
(a) CH3OH :- Alcohol
(b) HCHO:- Aldehyde
(c) CH3COOH:- Carboxyl
Solution 18.

Solution 19.
(i) Physical properties: The alkyl group determines the physical properties.
(ii) Chemical properties: The functional group is responsible for the chemical properties.
Solution 20.
The alkyl radical and the functional group are:
| Sr.No | Formula | Name of alkyl radical | Name of Functional group |
| a | CH3OH | Methyl | Alcohol |
| b | C2H5OH | Ethyl | Alcohol |
| c | C3H7CHO | Propyl | Aldehyde |
| d | C4H9COOH | Butyl | Carboxyl |
Solution 21.
(a) An alkyl group is obtained by removing one atom of hydrogen from an alkane molecule. Alkyl group is named by replacing the suffix ‘ane’ of the alkane with the suffix -yl.

Solution 22.

Solution 23.
(a) A homologous series is a group of organic compounds having a similar structure and similar chemical properties in which the successive compounds differ by a CH2 group.
(b) The difference in molecular formula of any two adjacent homologues is
(i) It differs by 14 a.m.u in terms of molecular mass.
(ii) It differs by three atoms. The kind of atoms it differs is one carbon and two hydrogen.
Miscellaneous Exercise
Solution 1.

Solution 2.

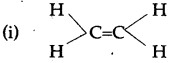
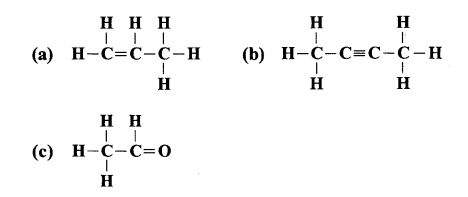
They both are unsaturated compound. The structure (i) contains double bond where as structure (ii) contains triple bond.
(b) Both the compounds undergo addition reactions.
Solution 3.

The Saturated hydrocarbons undergo substitution reactions whereas unsaturated hydrocarbons undergo addition reactions.
Solution 4.
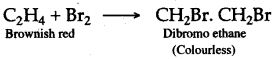
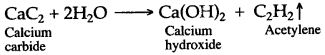
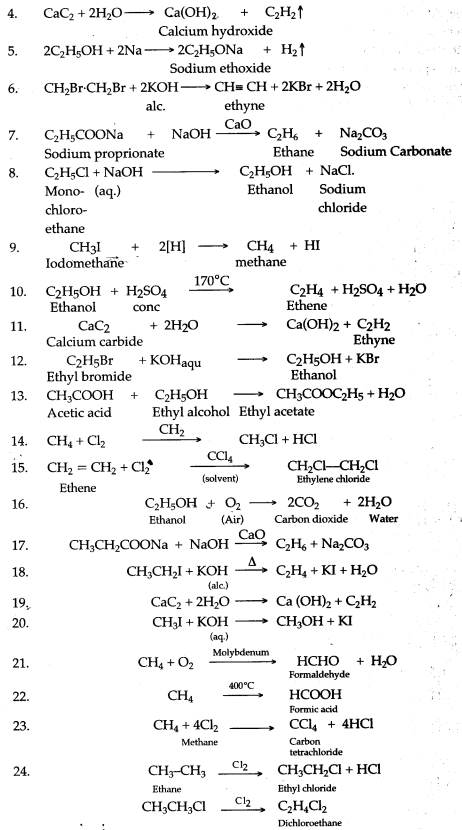
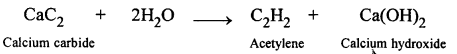
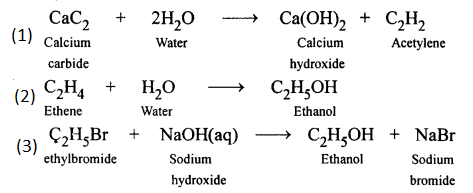
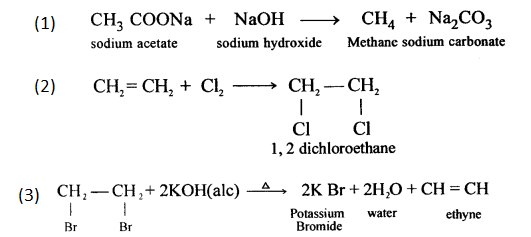
(a) CaC2 + 2H2O → Ca(OH)2 + C2H2
(b) When bromine in carbon tetrachloride is added to ethyne, the orange colour of the bromine disappears due to the formation of the colourless ethylene bromide.
![]()
Solution 5.
The alkanes form an (a) Homologous series with the general formula (b) CnH2n+2. The alkanes are (c) saturated (d) hydrocarbon which generally undergo (e) substitution reactions.
Solution 6.
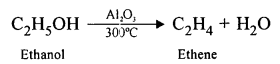
(a) The conversion of ethanol into ethene is an example of Dehydration.
(b) Converting ethanol into ethene requires the use of Conc. H2SO4.
(c) The conversion of ethene into ethane is an example of hydrogenation.
(d) The catalyst used in the conversion of ethene into ethane is commonly nickel.
Solution 7.
(a) Ethyne is a highly reactive compound than ethene because of the presence of a triple bond between its two carbon atoms.
(b) Ethene is a highly reactive compound than ethane because of the presence of a double bond between its two carbon atoms.
(c) Hydrocarbons such as alkanes undergo combustion reactions with oxygen to produce carbon dioxide and water vapour. Alkanes are flammable which makes them excellent fuels.
Methane for example is the principal component of natural gas.
CH4 + 2O2 → CO2 + 2H2O
Solution 1 (2004).
2C2H6 + 7O2 → 4CO2 + 6H2O
Solution 1 (2005).

Solution 1 (2006).
(a) IUPAC name: Propanal
Functional group: -CHO
(b) IUPAC name: Propanol
Functional group: -OH
Solution 1 (2007).

Solution 1a (2008).

Solution 1b (2008).

Solution 1c (2008).

Solution 1d (2008).
(i) Ethane undergoes substitution reaction.
(ii) Ethene undergoes addition reactions.
Solution 1e (2008).
(i) 2C2H6 + 7O2 → 4CO2 + 6H2O
(ii) Ethane can be oxidized as follows:
When a mixture of ethane and oxygen in the ratio 9:1 by volume is compressed to about 120 atm pressure and passed over copper tubes at 475K, ethyl alcohol is formed.
Solution 1f (2008).
(i) Pure acetic acid on cooling forms crystalline mass resembling ice and for this reason it is called glacial acetic acid.
(ii) When acetic acid reacts with alcohol, ester is formed.
![]()
Solution 2 (2004).

Solution 2 (2005).
(a) Ethanol
(b) Ethanoic acid
(c) Ethene
Solution 2 (2006).

Solution 2 (2007).
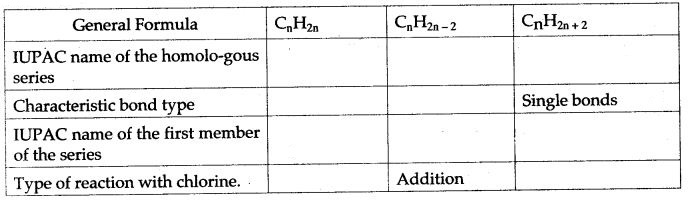
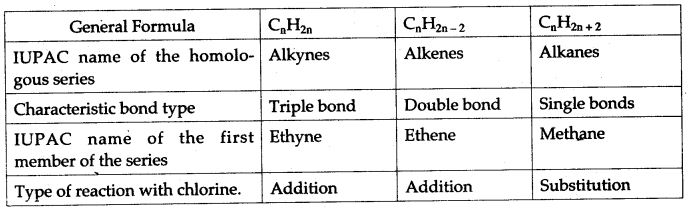
The homologous series of hydrocarbons are:
| General Formula | CnH2n | CnH2n-2 | CnH2n+2 |
| IUPAC name of the homologous series | Alkenes | Alkynes | Alkanes |
| Characteristics bond type | Double bond | Triple Bond | Single Bond |
| IUPAC name of the first member of the series | Ethene | Ethyne | Methane |
| Type of reaction with chlorine | Addition | Addition | Substitution |
Solution 3 (2005).

Solution 3 (2006).
Alkenes are the (a) homologous series of (b) unsaturated hydrocarbons. They differ from alkanes due to presence of (c) single bonds. Alkenes mainly undergo (d) addition reactions.
Solution 4 (2006).

More Resources for Selina Concise Class 10 ICSE Solutions


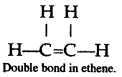
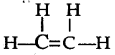
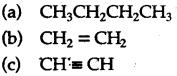
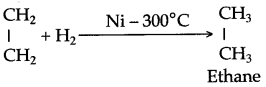
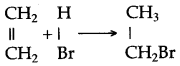
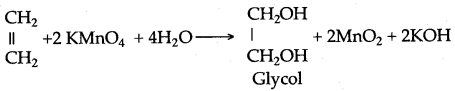
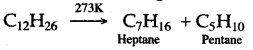
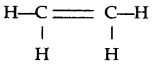
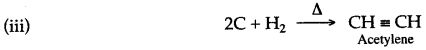
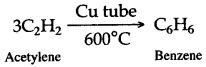
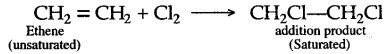
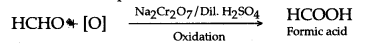
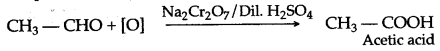

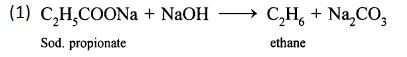

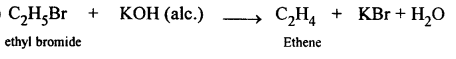
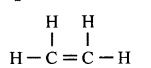
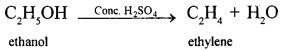
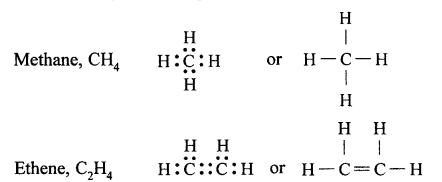
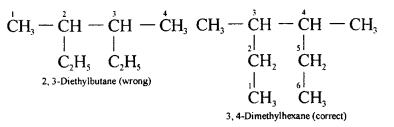
 ..
..