What is Atmospheric Pressure
Scientists discovered atmospheric pressure in the seventeenth century. This discovery uncovered an interesting fact that air actually has weight! The weight of the atmosphere presses down on the Earth’s surface and creates a pressure on it. The pressure at any point exerted by the weight of the air above it is called atmospheric pressure.
Atmospheric Pressure
- The pressure due to the Earths atmosphere can be considered as the result of the weight of the air acting on per unit area of the Earth’s surface.
- The atmospheric pressure is the pressure exerted by the atmosphere on the surface of the Earth as well as all objects on the Earth.
- At sea level, the atmospheric pressure is about 1.013 x 105 N m-2. This value is usually referred to as 1 atmosphere.
- Meteorologists express pressure in millibars. One millibar is 100 N m-2 or 100 Pa. Hence, 1 atmosphere is about 1013 millibars.
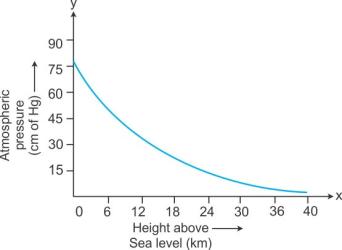
- The atmospheric pressure decreases slowly with altitude because the atmosphere becomes thinner at higher altitude. Figure illustrates the decreasing atmospheric pressure with altitude.
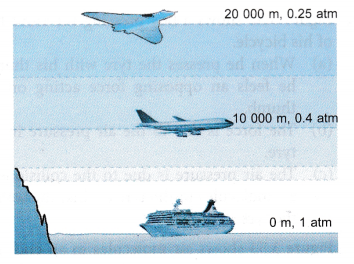
Variation of Atmospheric Pressure With Altitude
The altitude of a place is its height above sea level. The atmospheric pressure at a place depends on its altitude and decreases as we go up. We know that atmospheric pressure at a place is the force exerted by the weight of the air column above that place. As we go up, the length of the air column above us decreases. This means its weight decreases, and, therefore, the atmospheric pressure is smaller at higher places (than at sea level).
If the pressure of atmosphere is changed suddenly, the blood vessels in our body will burst due to the pressure of the blood and other fluids inside. This is why astronauts have to wear special pressurized suits—there is no air and, therefore, no air pressure in space.
Applications of Atmospheric Pressure
- Figure shows a person drinking with the help of a straw.
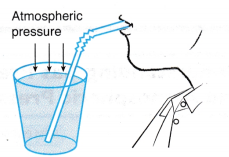 (a) He sucks the air in the straw and creates a partial vacuum in it.
(a) He sucks the air in the straw and creates a partial vacuum in it.
(b) The surrounding atmospheric pressure forces the drink into the straw and enters the mouth of the person. - Figure shows a worker carrying a piece of glass using a pair of rubber suction cups.
 (a) When he presses the suction cups against the glass, air is forced out of the cups creating a partial vacuum in it.
(a) When he presses the suction cups against the glass, air is forced out of the cups creating a partial vacuum in it.
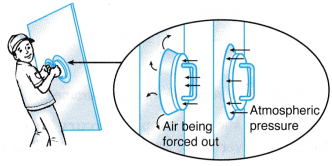
(b) The surrounding atmospheric pressure forces the rubber cups tightly against the smooth surface of the glass. - Figure shows a vacuum cleaner in use.
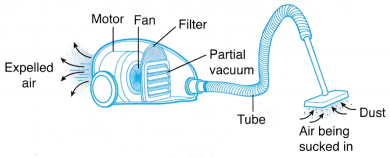 (a) The rotating fan expels air, creating a partial vacuum in the space in front of it.
(a) The rotating fan expels air, creating a partial vacuum in the space in front of it.
(b) The surrounding atmospheric pressure forces the air into the tube, carrying dust particles along with it.
(c) When the air passes through the filter, the dust particles are trapped by the filter so that the air expelled out of the vacuum cleaner is clean.
Instruments for Measuring Atmospheric Pressure
- Figure shows a simple mercury barometer made by inverting a long glass tube filled with mercury.
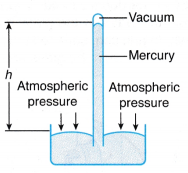 (a) Due to the inversion, a vacuum is created at the base of the tube and a column of mercury is supported by the atmospheric pressure.
(a) Due to the inversion, a vacuum is created at the base of the tube and a column of mercury is supported by the atmospheric pressure.
(b) The units for the measurement of atmospheric pressure can be in terms of centimetres of mercury (cm Hg) or pascal (Pa). Another unit of pressure is torr, where 1 torr is equal to 1 mm Hg.
(c) At sea level, the atmospheric pressure can support a column of mercury with a vertical height, h of 76 cm. Hence the atmospheric pressure, Patm can be calculated as:
 where, ρ is the density of mercury.
where, ρ is the density of mercury. - Figure shows a Fortin barometer which is essentially modified from a simple mercury barometer.
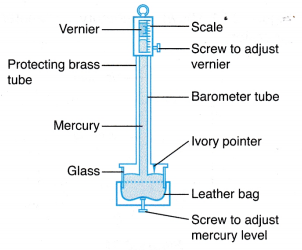 (a) The Fortin barometer has a metal scale with a vernier attached and a mirror behind the top of the mercury column to avoid parallax error when taking readings.
(a) The Fortin barometer has a metal scale with a vernier attached and a mirror behind the top of the mercury column to avoid parallax error when taking readings.
(b) With these features, the Fortin barometer measures the atmospheric pressure more accurately than the simple mercury barometer. - Figure shows an aneroid barometer. An aneroid barometer is a more convenient form of barometer because it does not use liquid and can be as small as a wristwatch.
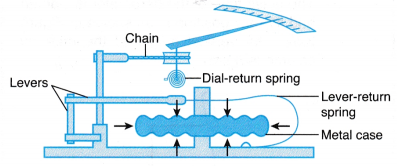 (a) Inside an aneroid barometer there is a partially evacuated metal case made of thin and flexible alloy.
(a) Inside an aneroid barometer there is a partially evacuated metal case made of thin and flexible alloy.
(b) A change of atmospheric pressure will cause the centre of the metal case to move in or out. This movement is magnified by a system of levers.
(c) A chain attached to the last lever rotates the pointer to show the atmospheric pressure measured.
Example 1. A mercury barometer in a physics laboratory shows a 732 mm vertical column of mercury.
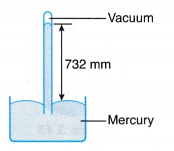
Based on this reading, calculate the atmospheric pressure in the laboratory in pascal.
[Density of mercury, ρ = 1.36 x 104 kg m-3; g = 9.8 N kg-1]
Solution:

Activity 1
Aim: To show the presence of atmospheric pressure.
Materials needed: A glass tumbler (with a smooth edge at the mouth, and without a rim), a piece of stiff cardboard (a little bigger than the mouth of the tumbler), and water. (It would be convenient to perform this activity over a wash basin or the kitchen sink.).
Method:
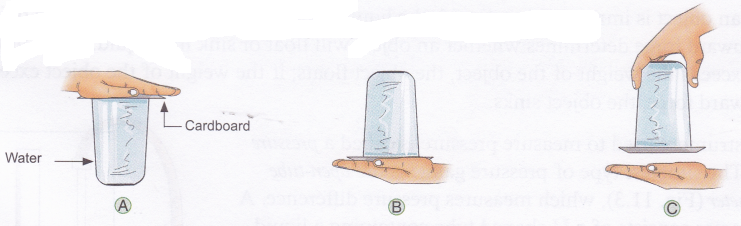
- Fill the tumbler with water to the brim.
- Cover the tumbler with the cardboard piece (figure A).
- Place the palm of your hand over the piece of cardboard, and quickly invert the tumbler (figure B).
- Slowly remove your hand supporting the piece of cardboard (Figure C).
Observation: You will observe that the cardboard piece will not fall.
Conclusion: Atmospheric pressure provides enough force to support a full glass of water.
Activity 2
Aim: To study atmospheric pressure using rubber suckers.
Materials needed: Rubber suckers.
Method: Take a rubber sucker and press it firmly to a smooth surface like a kitchen tile or a plain glass window. Try to pull it out.
 Observation: You will see that it is really difficult to pull the rubber sucker off the smooth surface.
Observation: You will see that it is really difficult to pull the rubber sucker off the smooth surface.
Conclusion: By pushing the rubber sucker against the smooth surface, you have created a partial vacuum, and the pressure of the air pressing on the outer surface of the sucker holds it in place.
Extension: Take a smooth stainless steel or ceramic plate and stick the rubber sucker on it. You can now hold the plate at any angle (horizontal, vertical, upside down, etc.) and try to pull the rubber sucker off the plate. You will find that the rubber sucker remains stuck to the plate regardless of the angle at which you hold the plate. This shows that air exerts pressure in all directions.