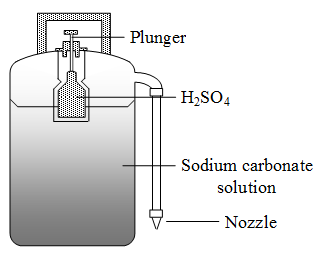
Fire extinguisher
The reaction between sulphuric acid and sodium carbonate or sodium hydrogencarbonate is utilized in the making of fire extinguisher as shown in Figure. A sealed glass bottle filled with dilute sulphuric acid is kept inside a container filled with an aqueous solution of sodium carbonate. In case of fire, the plunger is struck against a hard surface to break the bottle. As a result, sulphuric acid comes in contact with the sodium carbonate. The carbon dioxide gas which comes out is directed towards the fire.
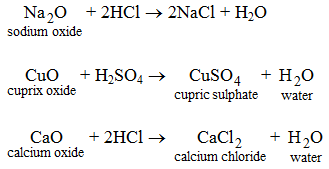
 Acids react with the oxides of metals to form salts and water.
Acids react with the oxides of metals to form salts and water.