SOLUTION AND SOLUBILITY
When some salt is added to water and stirred, the salt disappears. This is because the salt has dissolved in the water. Dissolving is a change where substances mix completely with the liquid they have been added to.
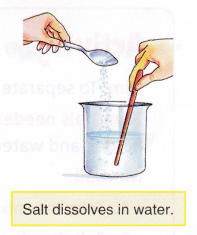 Not all substances dissolve in water. Only some substances like salt and sugar, dissolve in water and are known as soluble substances. Substances like chalk and sand do not dissolve in water and are known as insoluble substances.
Not all substances dissolve in water. Only some substances like salt and sugar, dissolve in water and are known as soluble substances. Substances like chalk and sand do not dissolve in water and are known as insoluble substances.
The substance that dissolves is called the solute and the substance in which the solute dissolves is called the solvent. The resulting mixture is called the solution.
Thus, solute + solvent = solution
E.g., sugar + water = sugar solution
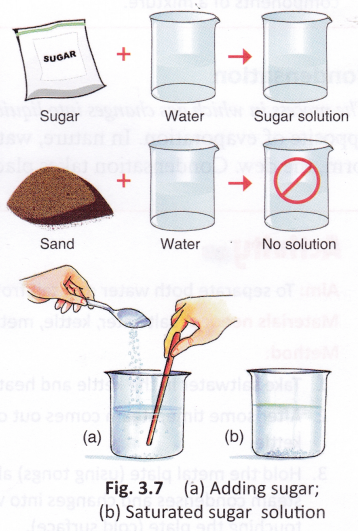 If we keep adding spoonfuls of sugar to water and stir the solution each time, what will happen after some time? We will notice some grains of sugar at the bottom of the solution. This shows that no more sugar can be dissolved. We say that the solution has become saturated.
If we keep adding spoonfuls of sugar to water and stir the solution each time, what will happen after some time? We will notice some grains of sugar at the bottom of the solution. This shows that no more sugar can be dissolved. We say that the solution has become saturated.
A saturated solution is the solution in which no more of the solute can be dissolved.
But what if we heat the solution? Can we then dissolve that ‘extra’ sugar present in the saturated solution?
Yes, we can increase the solubility of a solute by heating the solution. Solubility is the ability of a substance to get dissolved in a given liquid. The quantity of a substance that can dissolve in hot water is much more as compared to that in cold water.
Different materials have different solubility in water. Based on their solubility, materials can be soluble, insoluble, miscible, or immiscible.
Solid materials that dissolve in water are said to be soluble in water. For example, common salt and sugar. Solid materials that do not dissolve in water are said to be insoluble in water. For example, sand, wood, stone, chalk powder, and wax. Liquids that dissolve in water are said to be miscible in water. For example, alcohol, vinegar, lemon juice, honey, and glycerine. Liquids that do not dissolve in water are said to be immiscible in water. For example, kerosene, coconut oil, and diesel.
Some gases dissolve in water (e.g., carbon dioxide and oxygen). Oxygen dissolved in water is essential for the survival of aquatic organisms. Soft drinks have carbon dioxide dissolved in them. Gases like nitrogen, hydrogen, and helium are insoluble in water.
There are some other factors that increase the solubility of a solute.
Stirring: We can observe this by taking two glasses of water and adding a spoonful of sugar to each glass. Then we keep one glass undisturbed and stir the other. Sugar dissolves faster when the solution is stirred.
Solute in powdered form: We can observe this by taking two glasses of water and adding a whole sugar cube in one glass and powdered or crushed sugar cube in the other. Sugar in the powdered form dissolves first.
Different substances dissolve in different amounts of water while making a saturated solution.