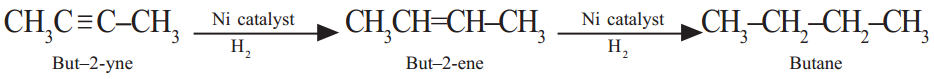Do Plants Breathe
Respiratory system in plants
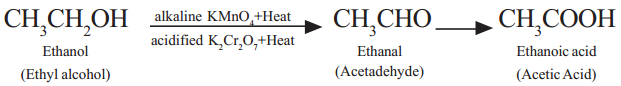
In plants, food that is manufactured in the form of starch needs to be broken down to release energy. So, respiration is a vital function in plants. It is represented as
Starch (Sugar) + Oxygen —► Energy + Carbon dioxide + Water
Plants respire through tiny holes or openings called stomata present on the underside of leaves. Stomata trap air (containing gases like oxygen and carbon dioxide) and the exchange of gases takes place inside plant cells.
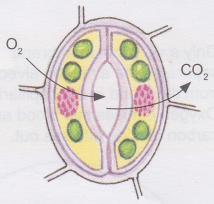
Even roots buried in the soil require oxygen for the plant to survive. Plant roots typically take in oxygen that is available in the small spaces between soil particles, and give off carbon dioxide. If roots cannot respire, they cannot produce the energy necessary to actively take up minerals from the soil. For proper root respiration, farmers plough or till the soil so that tiny air spaces are created around the soil particles.
Activity
Aim: To demonstrate that plants respire through stomata
Materials needed: Petroleum jelly, a leafy plant, e.g., geranium, dahlia, or rose
Method:
1. Keep the plant in the dark for a few days.
2. Choose two leaves and coat their underside with petroleum jelly.
3. Place the plant on a sunny window sill for a week and observe.
Observation The two leaves begin to die as time goes by.
Conclusion: This is because there is no way for air to enter.
How do plants/animals live in water?
Aquatic plants and animals survive in water by utilizing dissolved oxygen. The sources of dissolved oxygen are direct absorption from the atmosphere and release during photosynthesis by aquatic plants. Aquatic animals breathe through their skins, whereas many have gills. Aquatic plants directly exchange gases with the water surrounding their leaves, stems, and roots.
Activity
Aim: To show that heat is released during respiration
Materials needed: Two thermos flasks, seeds, formalin or carbolic acid, cottonwool, and two thermometers
Method:
1. Take two thermos flasks and mark them (1) and (2).
2. Take a glassful of seeds and soak them in water for more than 24 hours.
3. Divide the seeds into two equal groups.
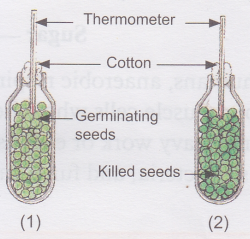
4. Boil one group of seeds and then wash them with dilute formalin or carbolic acid to prevent decay.
5. Put the live germinating seeds in flask (1) and the killed ones in flask (2).
6. Insert a thermometer in each flask and plug their mouths with cotton wool.
Observation: After a few hours, the thermometer in flask (1) shows a higher reading. The thermometer in flask (2) does not show any rise in temperature.
Conclusion: This indicates that the germinating seeds give out heat because they are alive and are respiring.
Aerobic and anaerobic respiration
Respiration is primarily of two types: aerobic and anaerobic.
1. Aerobic Respiration
The process of respiration that takes place in the presence of oxygen is called aerobic respiration. This results in the release of energy, and in the formation of carbon dioxide and water.
Aerobic respiration is represented by the equation:
Sugar + Oxygen —► Energy + Carbon dioxide + Water
Aerobic respiration is the most efficient form of respiration. The reaction involved in this process is similar to that of combustion or burning. However, there are some differences between the two.
Table Differences between aerobic respiration and combustion
| Aerobic Respiration | Combustion |
| 1. Energy is given out gradually. | 1. Energy is given out suddenly |
| 2. Energy is given out in the form that can be used by the organism. | 2. Energy is given out in the form of heat or light. |
| 3. Energy is stored inside the body in the form of ATP (adenosine triphosphate, energy-rich molecules) which can be used whenever needed | 3. Energy is not stored as ATP. |
2. Anaerobic Respiration
The process of respiration that takes place in the absence of oxygen is called anaerobic (“an” means “without”) respiration. This results in the release of energy, and in the formation of carbon dioxide and ethyl alcohol (an organic compound). It is represented by:
Sugar —► Energy + Carbon dioxide + Ethyl alcohol
In humans, anaerobic respiration can be carried out only for a short period of time. It occurs in our muscle cells when there is not enough oxygen supply. This happens when we are doing heavy work or exercise, running fast, etc. However, many microorganisms such as yeast, bacteria, and fungi can only respire anaerobically.
Activity
Aim: To demonstrate that oxygen is used up during aerobic respiration
Materials needed: Laboratory stand, a round-bottomed glass flask, KOH pellets, mercury, a glass tube, a beaker, and some seeds.
1. Take a few germinating seeds in the flask and arranged the apparatus as shown in the figure.
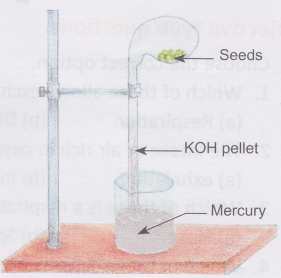
2. The glass tube is inverted into a beaker containing Seeds mercury.
3. KOH pellets are kept inside the glass tube as shown.
This helps to absorb the carbon dioxide so that it is not used up by the seeds.
4. Another similar setup without KOH pellets is set up alongside to act as control.
Observation: The mercury level rises in the setup with KOH pellets after a day. No change can be seen in the setup without KOH pellets.
Conclusion: When the oxygen in the apparatus is used up, it lowers the pressure inside the bulb, causing the level of mercury to rise up in the glass tube. This shows that oxygen is used by the seeds for aerobic respiration.