Explain the Cleansing Action Of Soaps And Detergents
The cleansing action of soaps and detergents:
- The cleansing action of both soaps and detergents results from their ability to lower the surface tension of water, to emulsify oil or grease and to hold them in a suspension in water.
- This ability is due to the structure of soaps and detergents.
- In water, a sodium soap dissolves to form soap anions and sodium cations. For example, the following chemical equation shows the ionisation of sodium palmitate.
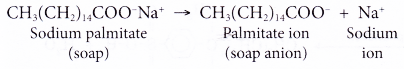
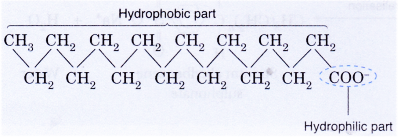
- A soap anion consists of a long hydrocarbon chain with a carboxylate group on one end.
- The hydrocarbon chain, which is hydrophobic, is soluble in oils or grease.
- The ionic part is the carboxylate group, which is hydrophilic, is soluble in water.
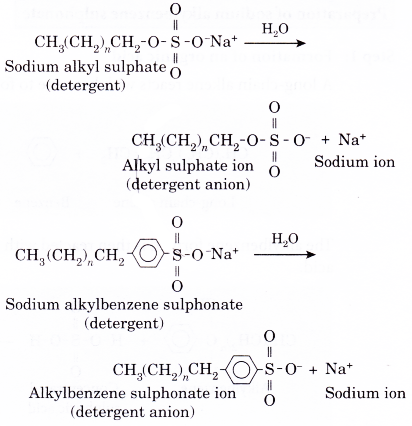
- In water, a detergent dissolves to form detergent anions and sodium cations. For example, the following chemical equations show the ionisation of sodium alkyl sulphate and sodium alkylbenzene sulphonate.
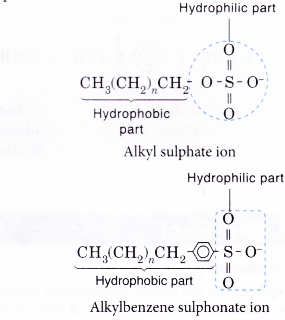
- Similarly, the anion part of a detergent also consists of a hydrophobic part and a hydrophilic part.
- The following explains the cleansing action of a soap or detergent.on a piece of cloth with a greasy stain.
- A soap or detergent anion consists of a hydrophobic part and a hydrophilic part.
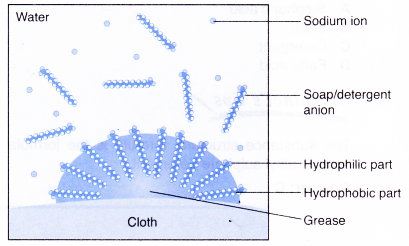
- Soap or detergent reduces the surface tension of water.
- Therefore, the surface of the cloth is wetted thoroughly.
- The hydrophobic parts of the soap or detergent anions are soluble in grease.
- The hydrophilic parts of the anions are soluble in water.
- Scrubbing or mechanical agitation helps to pull the grease away from the cloth and the grease is broken into smaller droplets.
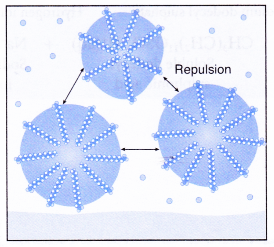
- Repulsion between the droplets causes the droplets to be suspended in water, forming an emulsion.
- Thus, the droplets do not coagulate or redeposit on the cloth.
- Rinsing washes away the droplets.
- A soap or detergent anion consists of a hydrophobic part and a hydrophilic part.
People also ask
- What is saponification in soap making?
- How do you make detergent?
- How are soaps and detergents different?
- What are the Advantages of Synthetic Detergents over Soap?
- Types of food additives and their functions
- What are the different types of medicine?
- The Existence of Chemicals
Soaps and detergents consist of a large hydrocarbon taill with a negatively charged head as shown in figures. The hydrocarbon tail is hydrophobic (water-hating or water repelling) and negatively charged head is hydrophilic (water-loving).
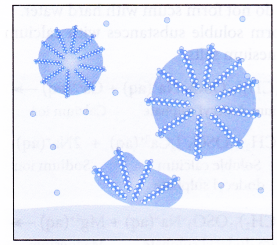
In aqueous solution, water molecules being polar in nature, surround the ions and not the hydrocarbon part of the molecule. When a soap or detergent is dissolved in water, the molecules associate together as clusters called micelles as shown in figure.
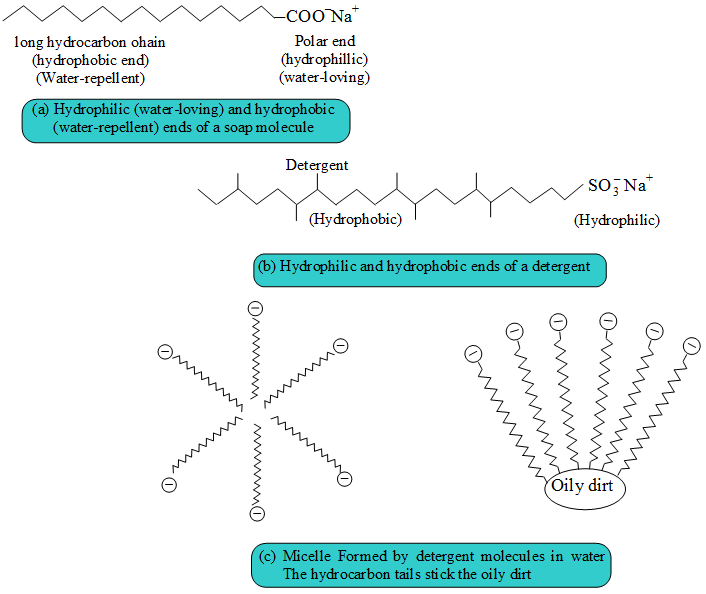
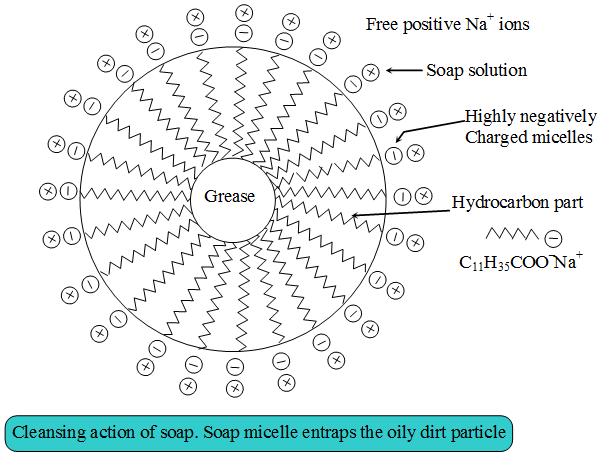
The tails stick inwards and the heads outwards.
In cleansing, the hydrocarbon tail attaches itself to oily dirt. When water is agitated (Shaken vigorously),the oily dirt tends to lift off from the dirty surface and dissociate into fragments.
This gives opportunity to other tails to stick to oil. The solution now contains small globules of oil surround by detergent molecules.
The negatively charged heads present in water prevent the small globules from coming together and form aggregates. Thus, the oily dirt is removed.
In the past, detergents caused pollution in rivers and waterbodies. The long carbon chain present in detergents used earlier, contained lot of branching. These branched chain detergent molecules were degraded very slowly by the micro-organims present in sewage discharge septic tanks and water bodies. Thus, the detergents persisted in water for long time and made water unfit for aquatic life. Nowadays, the detergents are made up of molecules in which branching is kept at minimum. These are degraded more easily than branched chain detergents.
Compare and contrast the effectiveness of the cleansing action of soap and detergent
- Although soap is a good cleaning agent, its effectiveness is reduced when used in hard water.
- Hard water contains a great amount of calcium and magnesium ions. These ions react with the soap to form an insoluble precipitate known as soap scum.


- The formation of soap scum reduces the amount of soap available for cleaning, thus causes wastage of soap.
- Soaps are only suitable for use in soft water. Soft water is water that contains little or no calcium and magnesium ions. Soaps do not form scum with soft water.
- The effectiveness of the cleansing action of soap is also reduced when used in acidic water.
(a) The hydrogen ions in acidic water react with the soap to form long-chain fatty acids.
(b) Long-chain fatty acids are insoluble in water due to their high relative molecular masses. This reduces the amount of soap available for cleaning. The effectiveness of the cleansing action of soap is thus reduced.
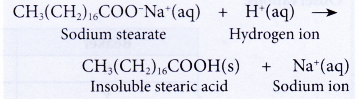
- Detergents do not form scum with hard water,
(a) They form soluble substances with calcium or magnesium salts.
(b) This means a detergent can still perform its cleansing action in hard water. Thus, a detergent is more effective than a soap in hard water.
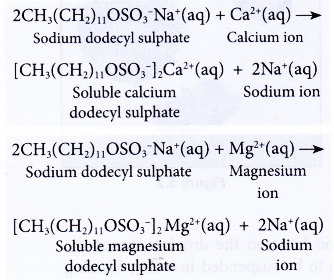
- Detergents do not form precipitates in acidic water. Thus, their cleansing action is not affected.
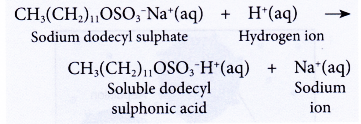
Effectiveness of the cleansing action of soap and detergent experiment
Aim: To compare the effectiveness of the cleansing action of soap and detergent in hard water.
Problem statement: Is the cleansing action of a detergent more effective than a soap in hard water?
Hypothesis: The cleansing action of a detergent is more effective than a soap in hard water.
Variables:
(a) Manipulated variables: Soap and detergent solutions
(b) Responding variable : The oily stains on a cloth
(c) Controlled variables : Volume and concentration of magnesium sulphate solution, volume and concentration of cleaning agent
Operational definition: The ability of a cleaning agent to remove oily stains on a cloth indicates that the cleaning agent is effective.
Materials: 5% soap solution, 5% detergent solution, 1 mol dm-3 magnesium sulphate solution, cloth with oily stains.
Apparatus: 100 cm3 beaker, 50 cm3 measuring cylinder.
Procedure:
- The soap and detergent solutions are prepared separately as shown in Figure.
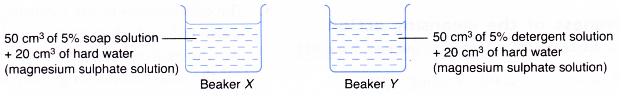
- A small piece of cloth with oily stains is dipped into each beaker.
- Each cloth is washed with the soap or detergent in each beaker.
- The cleansing action of the soap and detergent is observed, compared and recorded.
Observation:
| Beaker | Observation |
| X | Oily stains remain |
| Y | Oily stains disappear |
Discussion:
- The presence of magnesium ions in water increases the hardness of the water.
- The magnesium ions react with the soap to form soap scum, an insoluble precipitate.
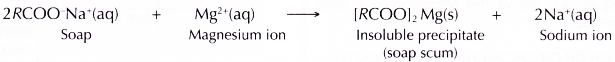
- The formation of soap scum reduces the amount of soap available for cleaning. The effectiveness of the cleansing action of soap is thus reduced.
- Detergents do not form scum with hard water. They form soluble substances with magnesium ions.
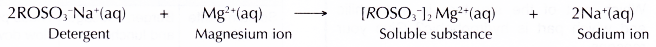
- Thus, a detergent is more effective than a soap in hard water.
Conclusion:
The cleansing action of a detergent is more effective than a soap in hard water. The hypothesis is accepted.