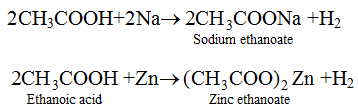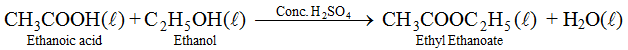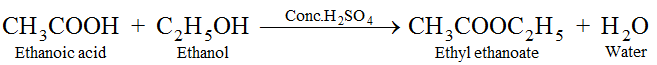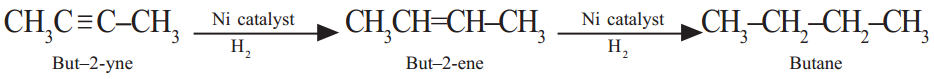Properties and Uses of Ethanol
Physical Properties of Ethanol :
(i) Pure ethanol is a colourless liquid.
(ii) It has a specific smell and burning taste
(iii) Its boiling point is 351 K which is higher than corresponding alkanes
(iv) It is soluble in water. i.e., it is miscible with water in all proportions.
Chemical properties of Ethanol :
(i) Dehydration : Ethanol. when heated with Conc. H2SO4 at 443 K or Al2O3 at 623 K undergoes dehydration, i.e. loses water molecule to from alkene.
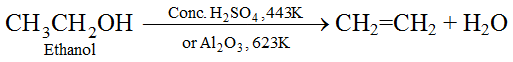
(ii) Reaction with Sodium : Alcohols are very weakly acidic. Ethanol reacts with sodium metal to form sodium ethoxide and hydrogen gas.
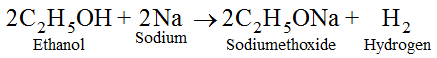
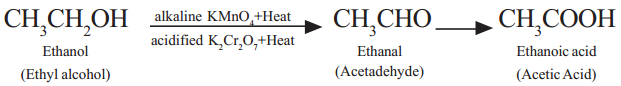
(iii) Oxidation with Chromic anhydride (CrO3) :
(iv) Oxidation with alkaline KMnO4 :
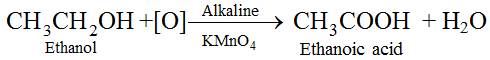
(v) Oxidation with acidified Potassium dichromate : Ethanol is oxidized to ethanoic acid with the help of acidified K2Cr2O7
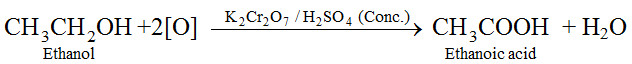
During this reaction, orange colour of K2Cr2O7 changes to green. Therefore, this reaction can be used for the identification of alcohols.
(vi) Esterification : Ethanol reacts with ethanoic acid in presence of concentrated H2SO4 to form ethyl ethanoate and water. The compound formed by the reaction of an alcohol with carboxylic acid is known as ester and the reaction is called Esterification. Esters are sweet fruity smelling compounds because they occur in fruits. They are used in ice creams, cold drinks and perfumes. The reaction takes place as follows.
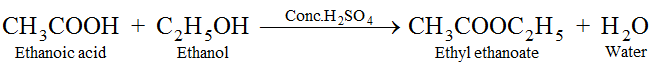
Conc. H2SO4 acts as dehydrating agent, i.e., it removes water formed otherwise ester formed will get hydrolysed.
(vii) Ethanol is highly inflammable liquid i.e., it catches fire very easily. It burns with blue flame in presence of oxygen to form carbon dioxide and water.
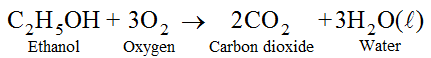
What is the use of ethanol?
Uses of Ethanol :
(i) Ethanol is present in alcoholic beverages such as beer, wine, whisky.
(ii) Ethanol is used as antiseptic for sterilising wounds.
(iii) Ethanol is used incough syrups. digestive syrups and tonics.
(iv) Ethanol is being mixed with petrol and is used as motor fuel. This mixture is called power alcohol.
(v) A mixture of ethanol and water has lower freezing point than water. This mixture is known as antifreeze and is used in radiators of vehicles in cold countries and at hill stations.
(vi) Ethanol is used for preparation of chloroform, iodoform, ethanoic acid, ethanal, ethyl ethanoate etc.
(vii) Ethyl alcohol is used as hypnotic (induces sleep).
People also ask
- What are carbon compounds?
- Chemical Properties of Carbon Compounds
- How are alkanes formed?
- What is an alkene in chemistry?
- What is an isomerism?
- What is alcohol and how is it made?
- How are carboxylic acids formed?
- How esters are formed?
- What are fats and oils?
- How palm oil is extracted?
- Order in Homologous Series
- What is the monomer of natural rubber?
- Which acid is used for coagulating rubber from latex?
- Classification of Hydrocarbons
- What is the homologous series of hydrocarbons?
- Properties and Uses of Ethanoic Acid