Selina Concise Chemistry Class 10 ICSE Solutions Chemical Bonding
APlusTopper.com provides step by step solutions for Selina Concise ICSE Solutions for Class 10 Chemistry Chapter 2 Chemical Bonding. You can download the Selina Concise Chemistry ICSE Solutions for Class 10 with Free PDF download option. Selina Publishers Concise Chemistry for Class 10 ICSE Solutions all questions are solved and explained by expert teachers as per ICSE board guidelines.
Download Formulae Handbook For ICSE Class 9 and 10
ICSE SolutionsSelina ICSE Solutions
Selina ICSE Solutions for Class 10 Chemistry Chapter 2 Chemical Bonding
Exercise Intext 1
Solution 1.
Atoms lose, gain or share electrons to attain noble gas configuration.
Solution 2.
(a) A chemical bond may be defined as the force of attraction between any two atoms, in a molecule, to maintain stability.
(b) The chemical bond formed between two atoms by transfer of one or more electrons from the atom of a metallic electropositive element to an atom of a non-metallic electronegative element.
(c) The chemical bond formed due to mutual sharing of electrons between the given pairs of atoms of non-metallic elements.
Solution 3.
Conditions for formation of Ionic bond are:
- The atom which changes into cation should possess 1, 2 or 3 valency electrons. The other atom which changes into anion should possess 5, 6 or 7 electrons in the valence shell.
- A high difference of electronegativity of the two atoms is necessary for the formation of an Ionic bond.
- There must be an overall decrease in energy i.e., energy must be released.
For this an atom should have low value of Ionisation potential and the other atom should have high value of electron affinity. - Higher the lattice energy, greater will be the case of forming an ionic compound.
Solution 4.
It will form a cation: M3+
M2(SO4)3
M(NO3)3
M3(PO4)3
M2(CO3)3
M(OH)3
Solution 5.
Atoms combine with other atoms to attain stable octet or noble gas configuration.
Solution 6.
Ionic compounds are generally formed between metals and non-metals as metals always lose electrons to form cations while non-metals gain electrons forming anions to complete their octet. These oppositely charged ions are held together by electrostatic force of attraction and hence results in an ionic compound.
Solution 7.
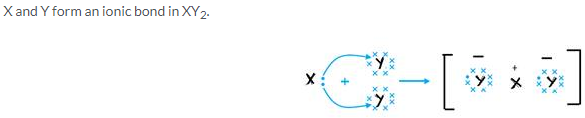
Solution 8.
(a) X has 7 electrons in its outermost shell and Y has only one electron in its outermost shell so Y loses its one electron and X gains that electron to form an ionic bond.
(b) The formula of the compound would be XY.
Solution 9.
Solution 10.
(a) Sodium atom and sodium ion
- Sodium atom has one electron in M shell while sodium ion has 8 electrons in L shell.
- Sodium atom is neutral while sodium ion is positively charged.
- Sodium atom is highly reactive while its ion is inert.
- Sodium atom is poisonous while sodium ion is non-poisonous.
(b) Chlorine atom and chlorine ion
- Chlorine atom has 7 electrons in its M shell while Chloride ion has 8 electrons in the same shell.
- Chlorine atom is neutral while chloride ion is negatively charged.
- Chlorine atom is highly reactive while its ion is inert.
- Chlorine gas is poisonous while chloride ion is non-poisonous.
Solution 11.
Fluoride ion is negatively charged while neon atom is neutral.
Solution 12.
(a) Transfer of electron(s) is involved in the formation of an electrovalent bond. The electropositive atom undergoes oxidation, while the electronegative atom undergoes reduction. This is known as a redox process.
Oxidation: In the electronic concept, oxidation is a process in which an atom or ion loses electron(s).
Zn → Zn2+ + 2e–
Reduction: In the electronic concept, the reduction is a process in which an atom or ion accepts electron(s).
Cu2+ + 2e–→ Cu
(b)
- Zn → Zn2+ + 2e– (Oxidation)
Pb2+ + 2e– → Pb (Reduction) - Zn → Zn2+ + 2e– (Oxidation)
Cu2+ + 2e–→ Cu (Reduction) - Cl2 + 2e–→ 2Cl– (Reduction)
2Br–→ Br2 + 2e– (Oxidation) - Sn2+→ Sn4+ + 2e– (Oxidation)
2Hg2+ + 2e–→ Hg2 (Reduction) - Cu+→ Cu2+ + e– (Oxidation)
Cu+ + e– → Cu (Reduction)
(c)
2K + Cl2→2KCl
- Oxidation: In the electronic concept, oxidation is a process in which an atom or ion loses electron(s).
K → K+ + e– - Reduction: In the electronic concept, the reduction is a process in which an atom or ion accepts electron(s).
Cl2 + 2e–→ 2Cl– - Oxidising agent
An oxidising agent oxidises other substances either by accepting electrons or by providing oxygen or an electronegative ion, or by removing hydrogen or an electropositive ion.
Cl2 + 2e–→ 2Cl– - Reducing agent
A reducing agent reduces other substances either by providing electrons or by providing hydrogen or an electropositive ion, or by removing oxygen or an electronegative ion.
K → K+ + e–
Exercise Intext 2
Solution 1.
(i) Both atoms should have four or more electrons in their outermost shells, i.e., non-metals.
(ii) Both the atoms should have high electronegativity.
(iii) Both the atoms should have high electron affinity and high ionisation potential.
(iv) Electronegativity difference between the two atoms should be zero or negligible.
(v) The approach of the atoms towards one another should be accompanied by decrease of energy.
Solution 2.
(a) A is a non-metal; B is a metal while C is a chemically inert element.
(b) BA
Solution 3.
(a) (i) E (ii) B
(b) C2D
(c) A and C are metals while B, D and E are non -metals.
Solution 3(2017).
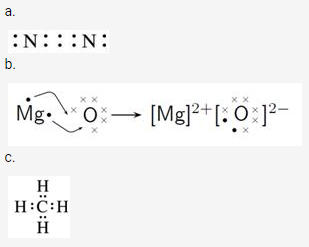
Solution 4.
(a) Ionic compounds are formed as a result of transfer of one or more electrons from the atom of a metallic electropositive element to an atom of a non-metallic electronegative element.
A polar covalent compound is the one in which there is an unequal distribution of electrons between the two atoms.
(b) Ionic compounds, made up of ions, are generally crystalline solids with high melting and boiling points.
They are soluble in water and good conductors of electricity in aqueous solution and molten state.
Covalent compounds, made up of molecules, can exist as soft solids or liquids or gases with low melting and boiling points. They are generally insoluble in water and poor conductors of electricity.
(c) Polar covalent compounds are formed between 2 non-metal atoms that have different electronegativities and therefore have unequal sharing of the bonded electron pair. Non-polar compounds are formed when two identical non-metals equally share electrons between them.
Solution 5.
(a) X+
(b) X will be a strong reducing agent as it will have the tendency to donate its valence electron.
Solution 6.
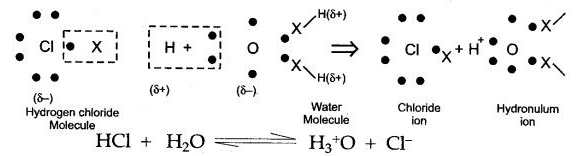
Covalent compounds are said to be polar when shared pair of electrons are unequally distributed between the two atoms. For example in HCl, the high electronegativity of the chlorine atom attracts the shared electron pair towards itself. As a result, it develops a slight negative charge and hydrogen atom develops a slight positive charge. Hence, a polar covalent bond is formed.
![]()
Solution 7.
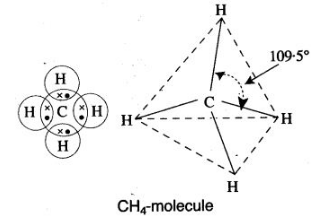
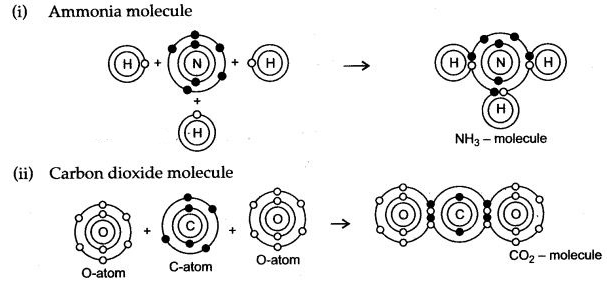
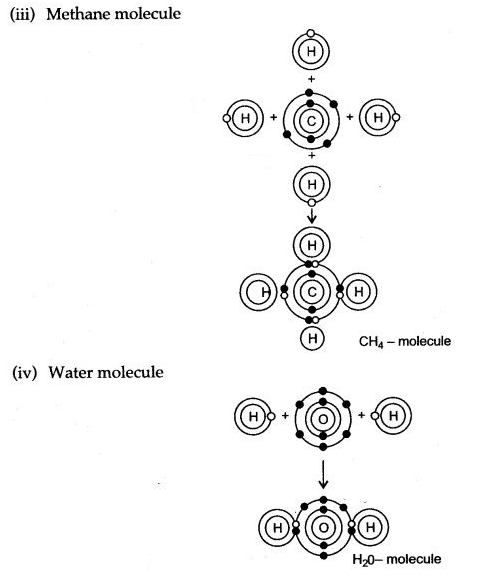
During the formation of a non-polar covalent bond between two similar atoms or dissimilar atoms, the atoms involved in sharing share the electrons equally. The molecule of methane has four carbon-hydrogen single covalent bonds. It is a non-polar covalent compound as the electrons are shared by the carbon and hydrogen atoms equally and hence the shared pair lies between the atoms at an equal distance from both carbon and hydrogen atom.
Solution 7.
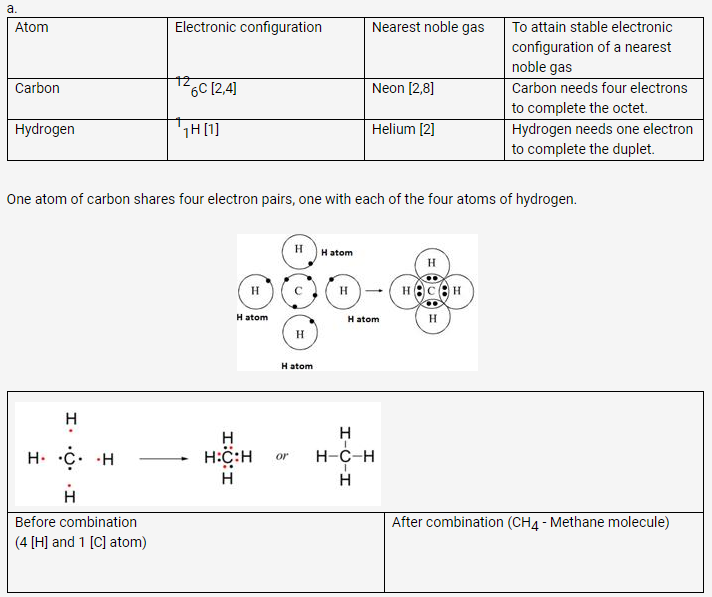
b. Methane is a covalent compound and is non-polar in nature. This is because the shared pair of electrons is equally distributed between the two atoms. So, no charge separation takes place and the molecule is symmetrical and electrically neutral.
Solution 8.
(a) Properties of Ionic Compounds:
- Ionic compounds usually exist in the form of crystalline solids.
- Ionic compounds have high melting and boiling points.
- Ionic compounds are generally soluble in water but insoluble in organic solvents.
- They are good conductors of electricity in the fused or in aqueous solution state.
(b) Properties of Covalent Compounds:
- The covalent compounds exist as gases or liquids or soft solids.
- The melting and boiling points of covalent compounds are generally low.
- Covalent compounds are insoluble in water but dissolve in organic solvents.
- They are non-conductors of electricity in solid, molten or aqueous state.
Solution 9.
(a)
- A reaction in which oxidation and reduction occur simultaneously is called an oxidation-reduction, or simply, a redox reaction.
- Redox reactions involve the transfer of electrons between two chemical species.
- The reaction in which electron is gained is called a reduction reaction and the reaction in which electron is lost is called oxidation reaction.
- The compound that loses an electron is said to be oxidized, the one that gains an electron is said to be reduced.
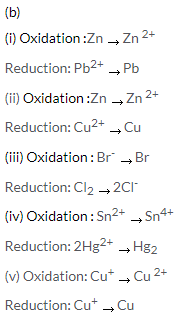
(c)
(i) Potassium undergoes oxidation as it loses an electron and forms a cation.
(ii) Chlorine undergoes reduction as it gains an electron and forms chloride anion.
(iii) Potassium acts a reducing agent and gets oxidised.
(iv) Chlorine acts an oxidizing agent and gets reduced.
Solution 9.
Solution 10.
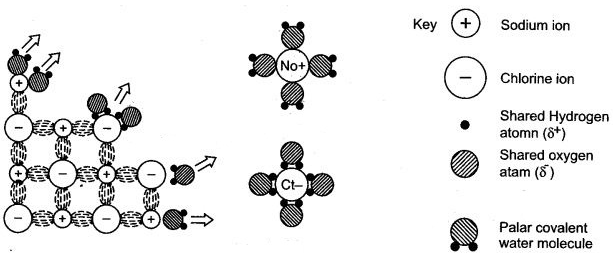
(a) Electrovalent compounds in the solid state do not conduct electricity because movement of ions in the solid state is not possible due to their rigid structure. But these compounds conduct electricity in the molten state. This is possible in the molten state since the electrostatic forces of attraction between the oppositely charged ions become weak. Thus, the ions move freely and conduct electricity.
(b) The atoms of covalent compounds are bound tightly to each other in stable molecules, but the molecules are generally not very strongly attracted to other molecules in the compound. On the other hand, the atoms (ions) in electrovalent compounds show strong attractions to other ions in their vicinity. This generally leads to low melting points for covalent solids, and high melting points for electrovalent solids.
(c) Electrovalent compounds dissolve in polar solvents like water because the forces of attraction between positive and negative charges become weak in water. But since covalent compound are made up of molecules, they do not ionize in water and hence do not dissolve in water.
(d) Since it takes a lot of energy to break the positive and negative charges apart from each other, the ionic compounds are so hard. But on applying stress, Ions of the same charge are brought side-by-side and so the opposite ions repel each other and crystal breaks into pieces.
(e) Since polar covalent compounds are made up of charged particles, they conduct electricity in aqueous solution.
Solution 10.
Dipole molecule is a molecule that has both, slight positive and slight negative charge.
For example, in HCl hydrogen has a slight positive charge and chlorine has a slight negative charge. The dipole moment of HCl molecule is 1.03 D and may be represented as:
![]()
Solution 11.
a.
i. Y = 9
ii. Z = 12
b. Ionic bond with molecular formula ZY2.
Solution 12.
| MgCl2 – Electrovalent compound | CCl4 – Covalent compound |
| They are hard crystalline solids consisting of ions. | These are gases or liquids or soft solids. |
| They have high melting and boiling points. | They have low melting and boiling points. |
| They conduct electricity in the fused or aqueous state. | They do not conduct electricity in the solid, molten or aqueous state. |
| These are soluble in inorganic solvents but insoluble in organic solvents. | These are insoluble in water but dissolve in organic solvents. |
Solution 13.
Potassium chloride is an electrovalent compound and conducts electricity in the molten or aqueous state because the electrostatic forces of attraction weaken in the fused state or in aqueous solution.
Polar covalent compounds like hydrogen chloride ionise in their solutions and can act as an electrolyte. So, both can conduct electricity in their aqueous solutions.
Solution 14.
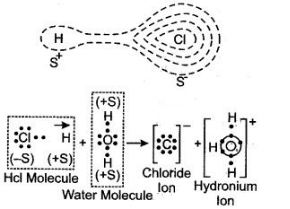
a. HCland NH3
b. HCl + H2O → H3O+ + Cl–
NH3 + H2O →NH4+ + OH–
Solution 15.
Formula of compound when combined with sulphur – MSFormula of compound when combined with chlorine –MCl2
Solution 16.
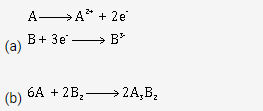
(c) If the compound formed between A and B is melted and an electric current is passed through the molten compound, then element A will be obtained at the cathode and B at the anode of the electrolytic cell.
Exercise 1
Solution 1.
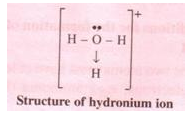
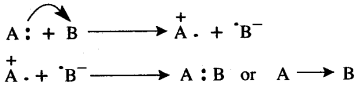
The bond formed between two atoms by sharing a pair of electrons, provided entirely by one of the combining atoms but shared by both is called a coordinate bond. It is represented by an arrow starting from the donor atoms and ending in the acceptor atom.
Conditions:
- One of the two atoms must have at least one lone pair of electrons.
- Another atom should be short of at least a lone pair of electrons.
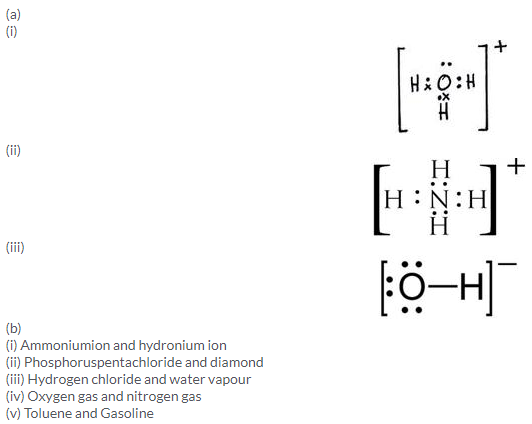
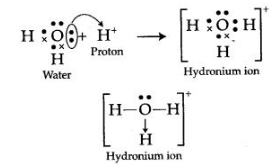
The two lone pair of electrons in the oxygen atom of water is used to form coordinate bond with the hydrogen ion which is short of an electron resulting in the formation of the hydronium ion.
H2O + H+ H3O+ Over here the hydrogen ion accepts one lone pair of electrons of the oxygen atom of water molecule leading to the formation of a coordinate covalent bond.
Solution 2.
A pair of electrons which is not shared with any other atom is known as a lone pair of electrons. It is provided to the other atom for the formation of a coordinate bond.
A pair of electrons which is shared between two atoms resulting in the formation of a covalent bond is called a shared pair.
Solution 3.
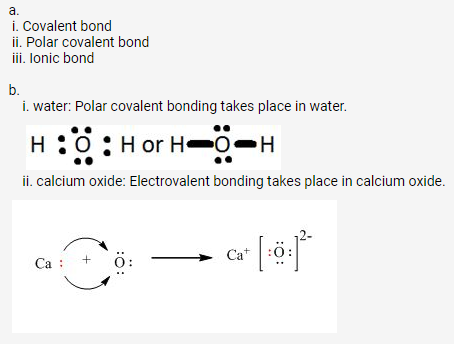
a. Polar covalent bond
b. Ionic bond
c. O and H are bonded with a single covalentbond and oxygen possesses a single negative charge in the hydroxyl ion.
d. Covalent bond
e. Coordinate bond
f. Electrovalentbond, dative bond (or coordinate bond) and covalent bond
Solution 4.
Solution 5.
Mg
Solution 6.
| Sodium | Phosphorus | Carbon | |
| Formula of chloride | NaCl | PCl5 | CCl4 |
| Nature of bonding | Ionic | Covalent | Covalent |
| Physical state of chloride | Solid | Solid | Liquid |
Solution 7.
a.
CaO- 1 calcium atom + 1 oxygen atom
Cl2 – 2 chlorine atoms
H2O – 2 hydrogen atoms + 1 oxygen atom
CCl4 – 1 carbon atom + 4 chlorine atoms
b.
Ca – will donate two electrons
O – will accept two electrons
Cl – will accept one electron, so two Cl atoms will share an electron pair.
C – will accept four electrons by sharing electrons pairs with hydrogen forming covalent bonds.
H – will donate one electron by sharing an electron pair with carbon.
Solution 8.
(a) Unequal, polar
(b) Middle, equally
(c) Electrovalent, electrostatic
Solution 9.
a.
- C
- C
- D
b.
- Y is getting reduced.
- Y is positive and it will migrate towards negative electrode that is cathode.
Solution 10.

Solution 1 (2004).
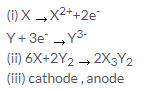
Solution 1 (2005).
(a) (i) C (ii) C (iii) D
(b) (i)reduced (ii) negative
(c) (i) H3O+ ions
(ii) Like dissolves like. Since carbon tetrachloride is non-polar and water is polar compound, carbon tetrachloride does not dissolve in water.
(iii) Solid
(iv) No as ionic bonds can only be made by transfer of electrons from a metal to non metal.
Solution 1 (2006).
(a) (i) B (ii) A
(b) (i) Reduction
(ii) Oxidation
(iii) Reduction
Solution 1 (2007).
(i) Ions
(ii) Electrons are shared between the atoms of two or more elements
(iii) Two
(iv) Magnesium is oxidized and chlorine is reduced
Solution 1 (2008).
(a)
(i) D
(b)
(i) Covalent bond
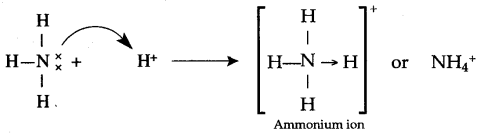
(ii) Coordinate bond.
Solution 1 (2009).
Solution 1 (2010).
a. Oxidation
b.
i. ionic bond
ii. covalent and oordinate bond
iii. covalent bond
Solution 1 (2011).
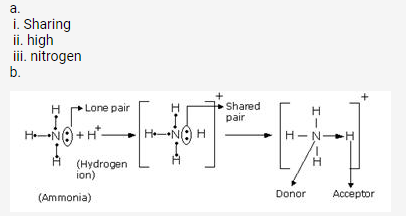
c. HCl is a covalent compound formed by sharing one electron between chlorine and hydrogen. Because chlorine is more electronegative than hydrogen, the shared pair of electrons shifts towards the chlorine atom. So, a partial negative charge (δ–) develops on chlorine and a partial positive charge (δ+) develops on hydrogen. Hence, the covalent bond is polar in nature.
Solution 1 (2012).
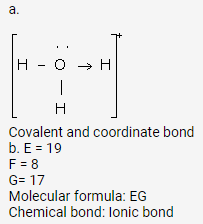
Solution 1 (2013).
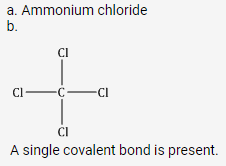
a. Dative or coordinate bond
b. B Ammonium chloride
c. C Are insoluble in water
d.
Carbon tetrachloride | Sodium chloride |
| It is insoluble in water but dissolves in organic solvents. | It is soluble in water but insoluble in organic solvents. |
It is a non-conductor of electricity due to the absence of ions. | It does not conduct electricity in the solid state but conducts electricity in the fused or aqueous state. |
Solution 1 (2014).
a. B
b. D
c. Ionisation
d. Their constituent particles are molecules. These exist as gases or liquids or soft solids because they have weak forces of attraction between their molecules.
More Resources for Selina Concise Class 10 ICSE Solutions
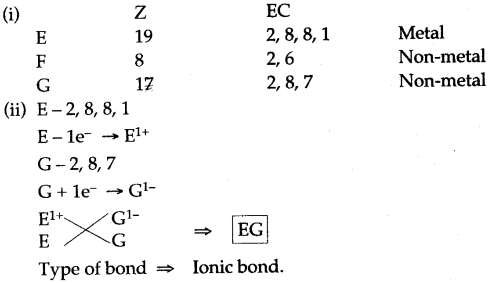
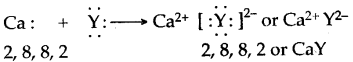
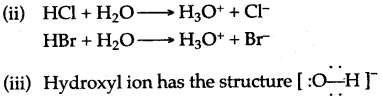
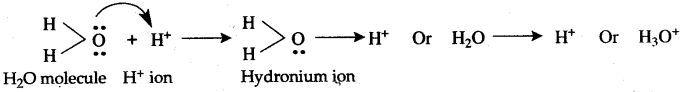
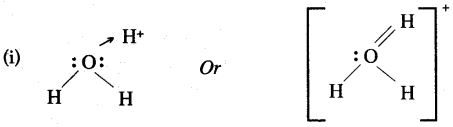
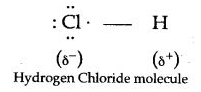
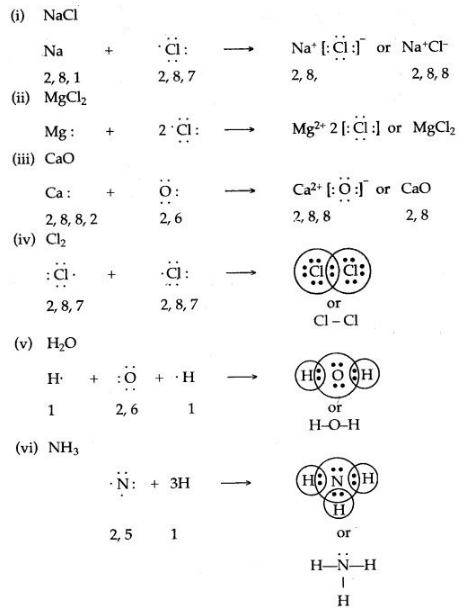
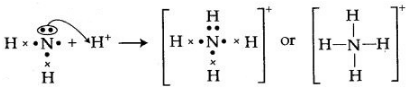
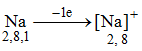 Thus, the electronic configuration of the Na+ ion is the same as that of neon which is the noble gas nearest to sodium in the periodic table.
Thus, the electronic configuration of the Na+ ion is the same as that of neon which is the noble gas nearest to sodium in the periodic table.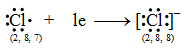 Thus, the chloride ion (Cl–) attains the configuration of the nearest noble gas, argon. [Valence electrons are shown by dots around the symbol.]
Thus, the chloride ion (Cl–) attains the configuration of the nearest noble gas, argon. [Valence electrons are shown by dots around the symbol.]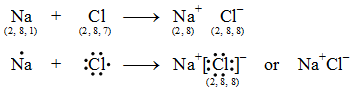 The formation of sodium chloride can be shown diagrammatically as in figure.
The formation of sodium chloride can be shown diagrammatically as in figure.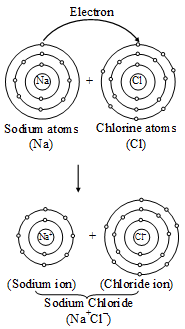 The force that holds Na+ and Cl– ions together is called an electrovalent bond. As this bond exists between ions, it is also called an ionic bond. An electrovalent bond is polar, i.e., the positive and negative charges are separated. Compounds containing such bonds are called’ electrovalent, or ionic, or polar compounds.
The force that holds Na+ and Cl– ions together is called an electrovalent bond. As this bond exists between ions, it is also called an ionic bond. An electrovalent bond is polar, i.e., the positive and negative charges are separated. Compounds containing such bonds are called’ electrovalent, or ionic, or polar compounds.