What is One Mole and How many Particles are in a Mole?
The Mole and the Number of Particles
- One mole is defined as the amount of substance that contains as many particles as the number of atoms in exactly 12 g of carbon-12, which is 6.02 × 1023 particles. The symbol of mole is mol.
- In chemistry, we use the unit ‘mole’ to represent the amount of substance containing 6.02 × 1023 particles.
1 mole of copper
= 6.02 × 1023 copper atoms
2 moles of copper
= 2 × 6.02 × 1023 copper atoms - The number of particles per mole (6.02 × 1023 mol-1) is determined experimentally and is known as the Avogadro constant or the Avogadro number.
The Avogadro constant (NA) is defined as the number of particles in one mole of a substance. - The particles in the substances can be atoms, molecules or ions. (Refer to the below table)
Type of substance Examples Atomic substances
(consist of atoms)All metal elements such as copper and zinc. All noble gases such as helium and argon. Certain non-metal elements such as carbon and silicon. Molecular substances (consist of molecules) Ail covalent compounds such as water and carbon dioxide. Certain non-metal elements such as hydrogen (H2), oxygen (O2), nitrogen (N2), fluorine (F2), chlorine (Cl2), bromine (Br2), iodine (I2), sulphur (S8) and phosphorus (P4). Ionic substances
(consist of ions)All ionic compounds such as sodium chloride and zinc bromide. - Therefore, the type of particles needs to be specified carefully.
(a) 1 mole of atomic substances contains 6.02 × 1023 atoms. For example, 1 mole of zinc contains 6.02 × 1023 zinc atoms.
(b) 1 mole of molecular substances contains 6.02 × 1023 molecules. For example, 1 mole of water contains 6.02 × 1023 H2O molecules.
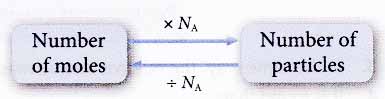
(c) 1 mole of ionic substances contains 6.02 × 1023 formula units. For example, 1 mole of sodium oxide contains 6.02 × 1023 formula units of Na2O. - We can find the number of particles in any number of moles of substances and vice versa using the following relationship.
People also ask
- What is the Relative Atomic Mass and Relative Molecular Mass of an Element?
- How do you Calculate the Molar Mass of a Substance?
- What is the Molar Volume of a Gas at STP?
- How do you know the Order of Elements in a Chemical Formula
- What is Empirical and Molecular Formula?
- How do you Write a Chemical Equation?
The Mole and the Number of Particles Problems with Solutions
1. Find the number of atoms in
(a) 0.4 mole of iron.
(b) 0.1 mole of oxygen gas.
Solution:
(a) Iron, Fe is an atomic substance.
Given the number of moles of iron = 0.4 mol
Number of iron atoms = number of moles × NA
= 0.4 × 6.02 × 1023
= 2.408 × 1023 atoms
(b) Oxygen gas, O2 is a molecular substance.
Given the number of moles of oxygen gas = 0.1 mol
Number of oxygen molecules = number of moles × NA
= 0.1 × 6.02 × 1023
= 6.02 × 1022 molecules
Based on its formula O2, each oxygen molecule contains two oxygen atoms.
So, 6.02 × 1022 oxygen molecules contain 2 × 6.02 × 1022 oxygen atoms, that is 1.204 × 1023 oxygen atoms.
2. How many moles ofwater contain 3.01 × 1022 water molecules?
Solution:
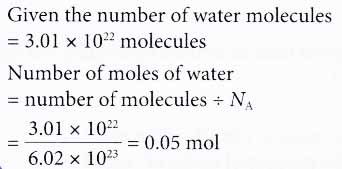
3. 0.3 mole of zinc bromide, ZnBr2 is dissolved in a beaker of water. Calculate the total number of ions present in the beaker.
Solution:
Zinc bromide, ZnBr2 is an ionic substance.
Given the number of moles of ZnBr2 = 0.3 mol
The number of formula units of ZnBr2 in the beaker
= number of moles × NA
= 0.3 × 6.02 × 1023
= 1.806 × 1023 formula units
Based on its formula, each formula unit of ZnBr2 consists of three ions, which are one zinc ion and two bromide ions.
Therefore, the total number of ions in the beaker
= 3 × number of formula units of ZnBr2,
= 3 × 1.806 × 1023
= 5.418 × 1023 ions
4. Which of the following contains 6.02 x 1023 atoms?
(A) 1 mol of nitrogen gas
(B) 1 mol of bromine gas
(C) 1 mol of carbon dioxide
(D) 1 mol of neon
Solution: D
6.02 x 1023 atoms is equivalent to 1 mol of atoms.
1 mol of nitrogen gas, N2 contains 2 mol of atoms.
1 mol of bromine gas, Br2 contains 2 mol of atoms.
1 mol of carbon dioxide, CO2 contains 3 mol of atoms.
1 mol of neon, Ne contains 1 mol of atoms Neon is a noble gas.
It is a monoatomic gas, unlike nitrogen, bromine and carbon dioxide gases.
Thus, 1 mol of neon contains 1 mol of atoms.