What is the Formula for Specific Heat Capacity?
Specific Heat Capacity
Figure shows water and cooking oil in similar pots and supplied with heat at the same rate.
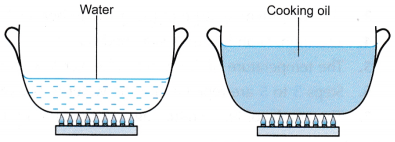 Which liquid heats up at a faster rate: water or cooking oil?
Which liquid heats up at a faster rate: water or cooking oil?
Such a deduction would be difficult to make because the water and cooking oil have different masses. A better comparison would be when both the water and cooking oil have the same mass.
- The specific heat capacity of a substance is the amount of heat that must be supplied to increase the temperature by 1°C for a mass of 1 kg of the substance.
- Specific heat capacity is expressed with units J kg-1 °C-1 or J kg-1 °K-1.
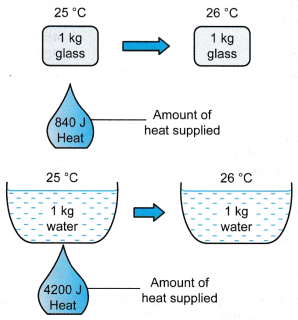
- The specific heat capacity of a type of glass is 840 J kg-1 °C-1. This means that 840 J of heat is required to raise the temperature of 1 kg of the glass by 1 °C.
Water has a specific heat capacity of 4200 J kg-1 °C-1 which is five times larger than the specific heat capacity glass.
Figure illustrates the difference in specific heat capacities of glass and water.
- Specific heat capacity, c can be calculated from the amount of heat supplied, Q to a mass, m of a substance with the increase in temperature, θ.

- Therefore, the quantity of heat absorbed or lost from a body is given by:
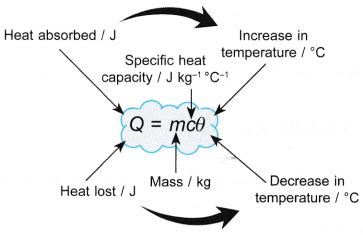
- It is important to relate the corresponding quantities and to use the correct units in the equation. Above figure illustrates this clearly.
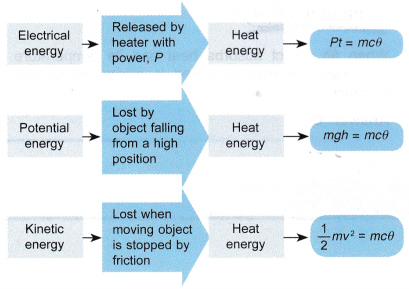
- Specific heat capacity is a physical property of a substance. Some substances have low specific heat capacities while some have higher specific heat capacities. Table lists the specific heat capacities of some common substances.
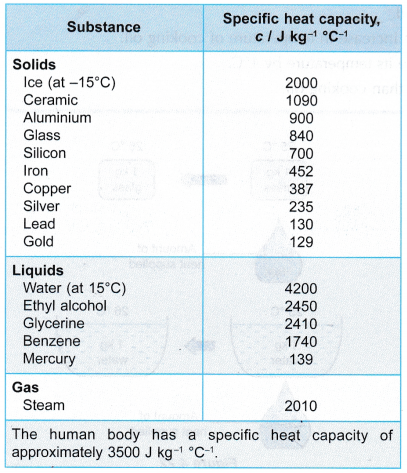
- Other forms of energy such as electrical energy, potential energy and kinetic energy can be converted to heat energy. This transformation can be summarised as shown in Figure.
People also ask
- What is Heat Capacity?
- What is meant by Latent Heat?
- What is the latent heat of fusion of Ice?
- What is latent heat of vaporization of Water?
Specific Heat Capacity of Water Experiment
Aim: To determine the specific heat capacity of water.
Material: Tap water
Apparatus: Polystyrene cup, immersion heater, thermometer, power supply, stirrer, beam balance or electronic balance, stopwatch
Method:
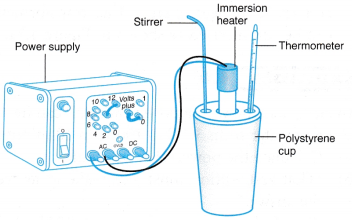
- The apparatus is set up as shown in Figure.
- The cup is filled with m g of water (for example, m = 200 g).
- The initial temperature of the water, θ1 is recorded.
- The heater is switched on. At the same time, the stopwatch is started.
- The water is stirred continuously so that its temperature is uniform.
- After t = 10 minutes, the heater Is switched off. The water is stirred and the highest temperature, θ2 is recorded in Table.
Results:

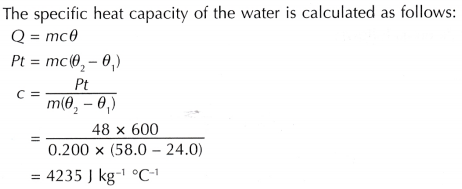
Analysis of Data:
Discussion:
- The polystyrene cup is preferred to a glass beaker because it is a poor conductor of heat. This will reduce the amount of heat lost to the surroundings. It has a small heat capacity. The heat absorbed by the cup is negligible.
- The value of the specific heat capacity of water obtained in this activity is larger than the standard value because some heat was lost to the surroundings during the heating of the water. In the calculation, all the heat supplied by the heater was assumed to be absorbed by the water.
Conclusion:
The specific heat capacity of water determined by the activity is 4235 J kg-1 °C-1
Specific Heat Capacity of Aluminium Experiment
Aim: To determine the specific heat capacity of aluminium.
Materials: Tissue paper, polystyrene sheet, a small amount of oil
Apparatus: Immersion heater, thermometer, power supply, beam balance, stopwatch, aluminium cylinder
Method:
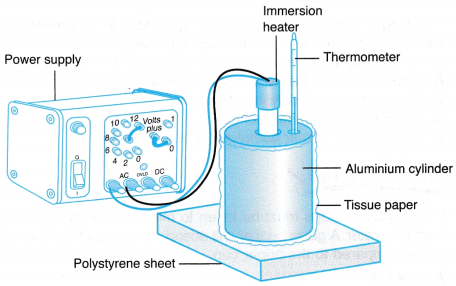
- The apparatus is set up as shown in Figure.
- The mass of the aluminium cylinder, m is determined using the beam balance.
- The initial temperature of the aluminium cylinder, θ1 is recorded in Table.
- The heater is switched on. At the same time, the stopwatch is started.
- After t = 10 minutes, the heater is switched off. The highest temperature, θ2 of the aluminium cylinder is recorded in Table.
Results:
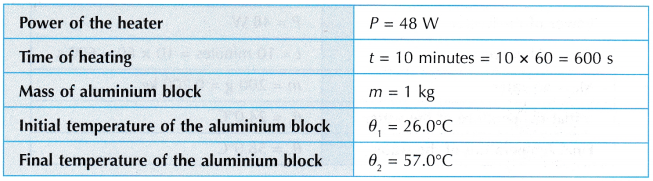
Analysis of Data:

Discussion:
- The tissue paper and polystyrene sheet were used to reduce the heat lost from the aluminium cylinder to the surroundings.
- A small amount of oil was put into the hole so that there will be good thermal contact between the bulb of the thermometer and the aluminium cylinder. This is to ensure that thermal equilibrium between the thermometer and the cylinder can be reached very quickly. The reading of the thermometer will then be equal to the temperature of the aluminium cylinder.
- The value of the specific heat capacity of aluminium obtained in this activity is slightly larger than the standard value because some heat was lost to the surroundings during the heating of the aluminium cylinder. In the calculation, all the heat supplied by the heater was assumed to be absorbed by the cylinder.
Conclusion:
The specific heat capacity of aluminium determined by the activity is 929 J kg-1 °C-1.
Applications of Specific Heat Capacity
- The physical meaning of specific heat capacity, c can be illustrated as follows:
(a) When two objects of equal mass are heated at equal rates, the object with the smaller specific heat capacity will have a faster temperature increase.
 (b) When two objects of equal mass are heated, to obtain the same temperature increase, more heat is needed to be supplied to the object with a larger specific heat capacity.
(b) When two objects of equal mass are heated, to obtain the same temperature increase, more heat is needed to be supplied to the object with a larger specific heat capacity.
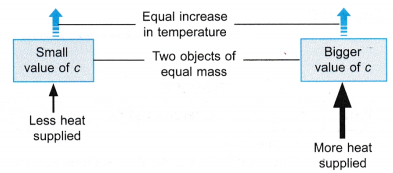
- When two hot objects of equal mass are left to cool down,
(a) the object with a smaller specific heat capacity will cool down at a faster rate,
(b) the object with a larger specific heat capacity will cool down at a slower rate. - Materials with small specific heat capacities are used in situations that are different from materials with large specific heat capacities.
- Cooking pot:
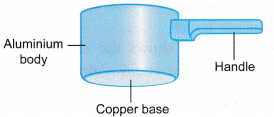
The body, base and handle of the cooking pot in Figure are made of materials with different specific heat capacities.
(b) Table shows the characteristics of the parts of the cooking pot.Part Characteristics Base Copper base.
Low specific heat capacity. Becomes hot very quickly. Enables quick cooking of the food in the pot.
High density. The heavier base ensures that the pot is stable and will not topple over easily.Handle Handle made of synthetic material.
Large specific heat capacity. Will not become too hot when heat is absorbed.
Poor conductor of heat. Very little heat from the body and contents of the pot is transferred to the hand of the person holding the pot.
Low density. Does not add very much to the total weight of the pot.Body Aluminium body.
Relatively low specific heat capacity. Becomes hot quickly.
Low density. Reduces the overall weight of the pot.
Does not react with the food in the pot. - The cooling system of a car engine:
(a) Water has a large specific heat capacity. It can absorb a large amount of heat without a high increase in temperature. Water is also readily available at low cost. This makes water very useful as a cooling agent in car engines and large machines that generate a lot of heat.
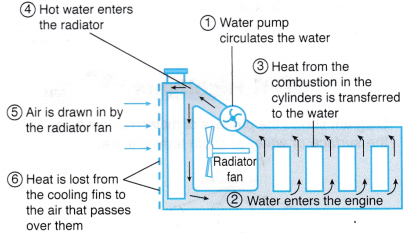
(b) Water is used to cool down internal combustion engines such as the car engine. Figure shows how heat is removed from the engine and lost to the surroundings using water as the surroundings using water as the cooling agent.
(c) A water pump circulates the water. Heat generated from the combustion of the petrol-air mixture is absorbed by the water that flows along the spaces in the engine walls. The hot water flows to the radiator where heat is lost to the cooler air that flows through the cooling
(d) The transfer of heat energy in the cooling system can be summarised as in Figure.

- Sea breeze and land breeze
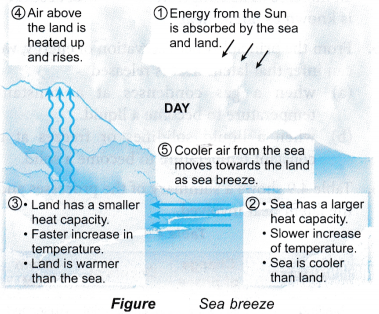
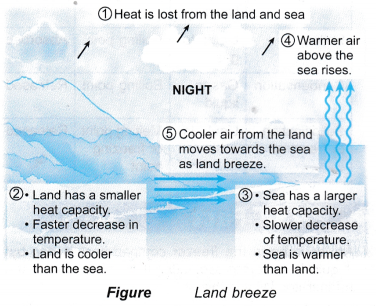
(a) Sea breeze is the natural breeze that blows from the sea towards the land during the day.
(b) Land breeze is the natural breeze that blows from the land towards the sea at night.
(c) They are caused by the sea having a bigger heat capacity than the land.
(d) Figure explain the formation of the sea breeze and land breeze. - Moderate climate:
(a) The presence of large masses of water such as lakes, the sea and the ocean can have an effect on the climate at a certain place.
(b) During the daytime in hot weather conditions, the water absorbs heat from the surroundings. This helps to reduce the temperature of the surroundings.
(c) During night-time, the water releases the heat absorbed. This prevents the temperature from dropping to very low values.
(d) In this way, areas near a large mass of water will have a smaller range of temperature changes and therefore, moderate climate conditions. - Factories with low ceilings:
Some factories which do not have large machinery are constructed with low ceilings. This reduces the volume of air inside the building. The smaller mass of air will have a smaller heat capacity. Less heat needs to be removed to cool down the air. This helps to reduce the air conditioning costs for the factory.
Specific Heat Capacity Example Problems with Solutions
Example 1. How much heat energy is required to raise the temperature of a 3 kg sheet of glass from 24°C to 36°C? [Specific heat capacity of glass = 840 J kg-1 °C-1]
Solution:
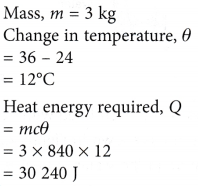
Example 2. Water in an ice maker of a refrigerator has a mass of 0.4 kg and a temperature of 22°C. What is the temperature of the water after 33 600 J of heat has been removed from it?
[Specific heat capacity of water = 4200 J kg-1 °C-1]
Solution:

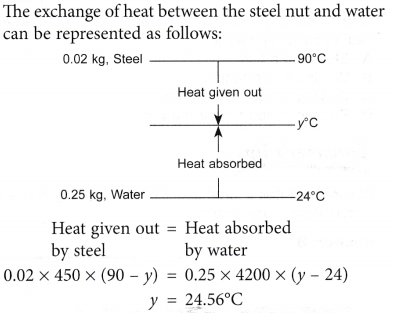
Example 3. A mechanic dropped a steel nut of mass 0.02 kg and temperature 90°C into 0.25 kg of water at 24°C in a polystyrene cup. What is the temperature when the steel nut and water have come to thermal equilibrium?
[Specific heat capacity of water = 4200 J kg-1 °C-1; Specific heat capacity of steel = 450 J kg-1 °C-1]
* Assume that the exchange of heat is between the steel nut and water only.
Solution:
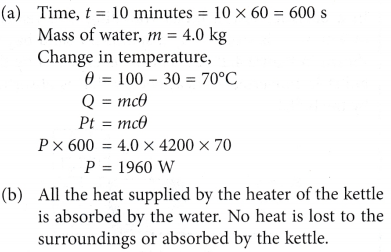
Example 4. An electric kettle with a power rating, P can heat up 4.0 kg of water from 30°C to 100°C in 10 minutes.
(a) Calculate the power, P of the kettle.
(b) What assumption must you make to arrive at the answer?
[Specific heat capacity of water = 4200 J kg-1 °C-1]
Solution:
Example 5. At a certain section of the Victoria Falls in Africa, water drops vertically through a height of 480 m.
(a) Explain why the water at the base of the waterfall has a temperature slightly higher than the water at the top.
(b) Estimate the maximum possible difference in the temperature between the water at the base and at the top of the waterfall. (Take g = 10 m s-2)
Solution:

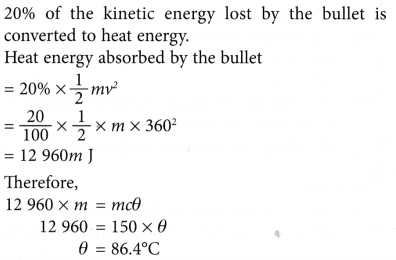
Example 6. In a ballistics test, a bullet travelling at a velocity of 360 m s-1 is stopped by a stationary sand bag as shown in Figure. 20% of the energy lost by the bullet is converted to heat energy that is absorbed by the bullet.
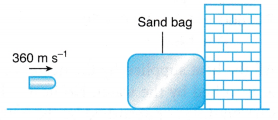
What is the increase in temperature of the bullet? [Specific heat capacity of the bullet = 150 J kg-1 °C-1]
Solution: