ICSE Solutions for Class 10 Physics – Specific Heat Capacity and Latent Heat
ICSE SolutionsSelina ICSE Solutions
APlusTopper.com provides ICSE Solutions for Class 10 Physics Chapter 10 Specific Heat Capacity and Latent Heat for ICSE Board Examinations. We provide step by step Solutions for ICSE Physics Class 10 Solutions Pdf. You can download the Class 10 Physics ICSE Textbook Solutions with Free PDF download option.
Download Formulae Handbook For ICSE Class 9 and 10

Short Answers
Question 1: What is heat? What is the S. I. unit of heat?
Answer: Heat is a form of energy, which when absorbed by a body makes it hot and when extracted from a makes it cold. It is also defined as the total kinetic energy possessed by all the molecules of a body. The S. I. unit of heat is joule/kg kelvin.
Question 2: Define the term calorie. How is it related with joule (the SI unit of heat)?
Answer: One calorie is the heat required to raise the temperature of 1 g of water from 14.5°C to 15.5°C
1 Calorie = 4.2 joule.
Question 3: Differentiate between heat and temperature.
Answer:
| Heat | Temperature |
| 1. It is a form of energy. | It is the sensation of hotness and coldness. |
| 2. Unit of heat is Joule. | Unit of temperature is °C or Kelvin. |
Question 4: What are other units of heat? Name and define them.
Answer: (i) Calorie: It is the quantity of heat required to raise the temperature of 1 gm of water by 1°C (more accurately the rise in temperature is taken from 14.5°C to 15.5°C).
(ii) Kilo calorie: It is the quantity of heat required to raise the temperature of 1 kg of water by 1°C. It is equivalent to 1000 calories.
Question 5: How is the heat capacity of a body related to the specific heat capacity of its substance?
Answer: Heat capacity of a body = Mass of the body × Specific heat capacity of its substance.
Question 6: State the condition for the flow of heat energy from one body to another.
Answer: Heat energy always flows from a body at a higher temperature to a body at a lower temperature.
Question 7: What are the factors on which the quantity of heat given to a body depends?
Answer: The quantity of heat given to body depends on:
(i) The mass of the body, (ii) The rise (or fall) in the temperature of the body, and (iii) Nature of the material of the body.
Question 8: m kg of a substance of specific heat capacity s J/kg °C is heated so that its temperature rises from θ1°C to θ2°C. Write down the expression for the heat Q supplied.
Answer: Heat supplied Q = mass × sp. heat capacity × rise in temperature
= m × s × (θ2 – θ1)
Question 9: Name the substance which has maximum specific heat capacity.
Answer: Water has the maximum specific heat capacity.
Question 10: Write two advantages of high specific heat capacity of water.
Answer: (i) Water is used as an effective coolant in radiator of a car.
(ii) Hot water bottles are used for fomentation.
Question 11: Does the specific heat capacity of a substance depend upon its mass and rise in temperature only?
Answer: No, for the same mass and for the same rise in temperature for different substance, the specific heat capacity is found to be different. Thus, specific heat capacity depends upon the nature of the substance also and so its is different for different substances.
Question 12: Write the approximate values of: (i) the specific latent heat of fusion of ice, (ii) the specific latent heat of vaporisation of steam.
Answer: (i) The specific latent heat of fusion of ice is 3,36,000 J/Kg.
(ii) The specific latent heat of vaporisation of steam is 22,60,000 J/kg.
Question 13: The specific latent heat of fusion of ice is 336 J/g. Comment on this.
Answer: The heat required to convert 1 g of ice from 0°C to 1 g water at 0°C is 336J.
Question 14: The farmers fill their fields with water in winter. Give reason.
Answer: The farmers fill their fields with water in winter to protect the crops from frost because water due to its high specific heat capacity does not allow the temperature in the surrounding area of plants to fall below 0°C.
Question 15: Why is specific heat capacity taken as a measure of thermal inertia?
Answer: We have H = ms Δθ or Δθ = (H/ms).
It follows that if s is more, Δθ will be small (for given values of H and m). Thus, for a given body, its specific heat capacity determines the change in temperature produced by a given quantity of heat. It is thus like mass in mechanics which determines the change in velocity (or the acceleration) produced by a given force. It is quite appropriate, therefore, to regard specific heat capacity as a measure of thermal inertia.
Question 16: Explain, why is water sprayed on roads in evening in hot summer?
Answer: Water has the highest specific heat capacity of 4.2 Jg-1 °C-1. Thus, water absorbs large amount of heat from the roads, but its own temperature does not rise much. Thus, on the whole the roads get cooled.
Question 17: Explain, why temperature in hot summer, falls sharply after a sharp shower?
Answer: Water has the highest specific heat capacity of 4.2 Jg-1 °C-1. Thus, when there is a sharp shower, the water evaporates. In doing so, it absorbs large amount of heat from surroundings, hence temperature falls sharply.
Question 18: Explain, why do sandy soils, get heated up quickly as compared to wet soils?
Answer: The specific heat capacity of sand is about five times less than water. Thus,when sun shines equally on sandy soil and wet soil, the sandy soil gets heated up rapidly as compared to wet soil. It is because water absorbs large amount of heat energy, but its temperature does not rise sufficiently.
Question 19: Explain, why water is considered as best liquid for quenching thirst?
Answer: Water has the highest specific heat capacity of 4.2 Jg-1 °C-1 and hence it can absorb large amount of heat energy, without rising in temperature sufficiently. Thirst is the natural signal, when body produces more heat energy than required. Thus, water is ideal for quenching thirst, because it can absorb large amount of heat energy.
Question 20: Explain, Why is it advisabile to pour cold water over burns, caused on human body, by hot solids?
Answer: Water has the highest specific heat capacity of 4.2 Jg-1°C-1. Thus, it can extract large amount of heat energy rapidly from the site of burn and hence gives a lot of relief.
Question 21: Explain, why does a wise farmer water his fields, if forecast is forst?
Answer: The frost can seriously damage the leaves and fruit of plants. When a farmer waters his fields, during, night, this water gives large amount of heat energy, because 1 g of water will liberate 4.2 J of energy for every 1°C fall in temperature. Thus, the air around the field is saturated with heat energy and its temperature does not fall below 0°C. Thus, no frost is formed.
Question 22: Explain, why are big tubs of water kept in underground cellars for storing fresh fruit and vegetables in cold countries.
Answer: During sub zero temperatures, the water within the cells of fruits and vegetables freezes and hence damages them. To avoid such a damage the huge water tubs are kept. The water gives off 4200 J of heat energy for every one kg for 1°C fall is temperature. Thus, it does not allow the temperature of cell to fall below 0°C and hence there is no damage to fresh fruits or vegetables.
Question 23: Explain, why is water used as a coolent in motor car radiators?
Answer: Water has the highest specific heat capacity of 4.2 Jg-1 °C-1 and its temperature does not rise beyond 100°C. Thus, it can absorb large amount of heat from the working engine, which is then radiated out through radiator Thus, on low temperature the engine works efficiently.
Question 24: Differentiate between heat capacity and specific heat capacity.
Answer: Heat capacity: It is the amount of heat energy required to raise the temperature by 1°C. Specific heat capacity: It is defined as the heat capacity per unit mass of that body.
Question 25: Define specific latent heat of vaporization of a substance.
Answer: Specific latent heat of vapourization is the quantity of heat required to convert unit mass of a substance from liquid to vapour state without change of temperature.
Question 26: Name two factors on which the heat absorbed or given out by a body depends.
Answer: The mass and specific heat capacity are two factors on which the heat absorbed or given out by a body depends.
Question 27: Explain why water is used in hot water bottles for fomentation and also as a universal coolant.
Answer: Hot water bottles are used for fomentation since since water does not cool quickly due to its large specific heat capacity.
Water is used as an effective coolant because of its large specific heat capacity due to which it can extract more heat.
Question 28: Specific heat capacity of substance A is 3.8 J g-1 K-1 whereas the specific heat capacity of substance B is 0.4 J g-1 K-1. Which of the two is a good conductor of heat? How is one led to this conclusion?
Answer: Specific heat capacity ‘A’ 3.8 J/g/K. Specific heat capacity ‘B’ 0.4 J/g/K
‘B’ is a good conductor of heat.
Since specific heat capacity is the energy required to raise the temp. of 1 g of a substance 1°C. So less heat energy passes through the substance.
Question 29: If substances A and B are liquids then which one would be more useful in car radiators?
Given: Specific heat capacity’A’ 3.8 J/g /K. Specific heat capacity ‘B’ 0.4 J/g /K.
Answer: ‘A’ which has more specific heat will be preferable for car radiators as it will act as coolant.
Question 30: How is the heat capacity of the body related to its specific heat capacity?

Question 31: A certain amount of heat Q will warm 1 g of material X by 3° C and 1 g of material Y by 4°C. Which material has a higher specific heat capacity?
Answer: X has higher specific heat capacity.
Heat given = Heat Taken.
Let mass of water used be m, then
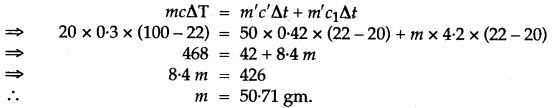
Question 32: Give one example where high specific heat capacity of water is used as a heat reservoir.
Answer: For fermentation purpose in hot water bottles.
Question 33: Give one example where high specific heat capacity of water is used for cooling purposes.
Answer: In car radiators.
Question 34: Name the liquid which has the highest specific heat capacity.
Answer: Water has the highest specific heat capacity.
Question 35: Name two factors on which the heat absorbed or given out by a body depends.
Answer: The mass and specific heat capacity are two factors on which the heat absorbed or given out by a body depends.
Question 36: An equal quantity of heat is supplied to two substances A and B. The substance A shows a greater rise in temperature. What can you say about the heat capacity of A as compared to that of B?
Answer: Heat capacity of substance B is more than substance A.
Question 37: Is it possible to condense the water formed, back to ice by adding ice at 0°C. Explain with reason.
Answer: 1 gm water will contain more heat in it. Because it contains latent heat 80 cal.
Question 38: What is ‘Calorimetry’?
Answer: The measurement of the quantity of heat is termed as colorimetry.
Question 39: State the principle of calorimetry.
Answer: According to the principle of calorimetry, if two bodies at different temperatures are mixed
together (or kept in contact), heat flows from a body at higher temperature to a body at lower temperature till both the bodies attain the same temperatue. If their is no heat lost to the surroundings, then
The heat lost by the hot body = The heat gained by the cold body.
Question 40: Give two reasons as to why copper is preferred over other metals for making calorimeters.
Answer: Copper is preferred over other metals for making calorimeters because:
(i) It has a low specific heat capacity.
(ii) It takes negligible amount of heat from its contents to attain the temperature of the contents.
Question 41: Some heat is provided to a body to raise its temperature by 25°C. What will be the corresponding rise in temperature of the body as shown on the Kelvin scale?
Answer: Increase in Kelvin will be 25K.
Question 42: If, in a central heating system, steam enters a radiation pipe at 100°C and water leaves the radiation pipe at 100°C, can this radiation pipe heat a room? Give an explanation for your answer.
Answer: Yes, because steam at 100°C gives out its latent heat of vaporisation to condense to water ut the same temperature of 100°C.
Question 43: Ice-cream at 0°C feels colder than water at 0°C. Give reason for this observation.
Answer: We know that we need to supply about 340 joule of heat per g, to convert ice at 0°C, into water at 0°C. It follows that that ice-cream, at 0°C, will draw more heat (about 340 joule more per gram) from burbody than water at 0°C. It, therefore, feels colder than water at 0°C.
Question 44: Bottled drinks are cooled more effectively, when surrounded by lumps of ice than iced water.
Answer: Every 1 kg of ice at 0°C absorbs 336,000 J of heat energy to form water at 0°C. As ice can extract 336,000 J of heat energy, more than water at 0°C, therefore it cools the bottled drinks more effectively.
Question 45: Write an expression for the heat energy liberated by a hot body. Expression for the heat energy liberated by hot body = mcΔQ
i.e., H = mcΔQ
where m is mass, c is specific heat capacity, ΔQ is the change in temperature.
Question 46: 1 kg of water freezes to form ice at 0°C. What amount of heat is withdrawn?
Answer: 3,36,000 J.
Question 47: Why does the heat supplied to a substance during its change of state not cause any rise in its temperature?
Answer: During the state change heat supplied increases potential energy of molecules as distance between molecules increases work is done by heat supplied against attractive force.
Question 48: What energy change would you Expect to take place in the molecules of a substance when it undergoes:
(i) a change in its temperature?
(ii) a change in itsstate without any change in its temperature?
Answer: (i) Inter molecular space changes.
(ii) Intermolecular space increases.
Question 49: Ice is more effective in cooling than the ice-water. Explain.
Answer: Each 1 g of ice absorbs nearly 336 J of heat when melts to water at 0°C. Hence, it is more effective in cooling than the ice-water.
Question 50: Why does atmospheric temperature fall after hail storm?
Answer: Ice has the highest sp. latent heat of fusion of 336,000 Jkg-1. Thus, when hails melt, they absorb large amount of heat energy from surroundings. Thus, atmospheric temperature drops.
Question 51: 1 kg of steam condenses to water at 100°C. How many joules of heat energy is liberated?
Answer: 22,60,000 J.
Question 52: What happens to the average kinetic energy of the molecules as ice melts at 0°C?
Answer: Average Kinetic Energy of molecules increases.
Question 53: Why do doctors advise to put a strip of wet cloth on the forehead of a person having high fever (temperature)?
Answer: This depends upon the fact that water in the process of evaporation takes away heat equivalent to latent heat from the patient’s body and thus lowers the temperature of his body.
Question 54: Explain the meaning of the term latent heat. State its S. I. unit.
Answer: The heat absorbed or liberated by a substance during its change of state at a constant temperature is called latent heat. Its S. I. unit is J/kg.
Question 55: What happens to the heat supplied to a substance when the heat supplied causes no change in the temperature of the substance?
Answer: This heat supplied is used in the change of state. This heat is known as Latent heat.
Question 56: When 1 g of ice at 0 °C melts to form 1 g of water at 0 °C then, is the latent heat absorbed by the ice or given out by it?
Answer: Latent heat is absorbed by the melting ice.
Question 57: Which contains more heat: 1 g water at 100°C or 1 g steam at 100°C? Give reason.
Answer: 1g steam at 100°C contains more heat than 1g water at 100°C. The reason is that lg steam at 100°C, when converts to 1 g water at 100°C, liberates 2260 J heat.
Question 58: Give one consequence of the high specific latent heat of fusion of ice.
Answer: Due to high specific latent heat of fusion of ice, snow on the mountains does not melt all at once but changes into water slowely as it gets heat from the sun.
Question 59: Why does weather become pleasant when it starts freezing in cold countries?
Answer: Ice has the highest sp. latent heat of fusion of 336,000 jkg-1. Thus, every 1 kg of water at 0°C, on freezing releases 336,000 J of heat energy. As enormous amount of heat energy is released in atmosphere, therefore weather becomes pleasant.
Question 60: Why water get cooled in a ‘Surahi’ in hot season?
Answer: ‘Surahi’ is made of clay which is porous (being not glazed) and so water oozes out (creeps out) and wets its entire surface. Form its outer surface, water get evaporated taking latent heat needed for evaporation from inside water as well as surrounding air and so the temperature of inside water falls.
Question 61: Why are athletes advised to put on extra clothes after competing on event?
Athletes perspire profusely after competing in an event. The latent heat of evaporation of the ‘sweat’ is withdrawn from the body itself and therefore, cools the body. Hence, if they do not put on extra clothes, they are likely to catch cold.
Question 62: Define the term ‘specific latent heat of fusion’ of a substance.
Answer: Specific latent heat of fusion: The specific latent heat of fusion of a substance is the heat energy released when a unit mass of substance converts from liquid to solid state without the change in temperature.
Question 63: The specific latent heat of vaporisation of steam is 2260 J/g. Comment on this.
Answer: The heat required to convert 1 g of water at 100°C to 1 g steam at 100°C is 2260 J.
Question 64: Define specific latent heat of vaporization of a substance.
Answer: Specific latent heat of vaporization is the quantity of heat required to convert unit mass of a substance from liquid to vapour state without change of temperature.
Question 65: Explain the statement; “The specific latent heat of vaporization of wafer is 2260 × 103 J/kg”.
Answer: It means that at 100°C, 2260 × 103 J of heat energy is absorbed by 1 kg of water to convert it into steam at 100°C, or 1 kg of steam at 100°C liberates 2260 × 103 J of heat energy in converting into 1 kg of water at 100°C.
Question 66: Why do we feel much comfortable when we sit under a moving fan especially when our body is sweating?
Answer: The reason is that sweat from our body evaporates faster under a moving fan due to rapid movement of air and so a cooling sensation is caused which makes us more comfortable.
Question 67: Explain, why no tracks are left on the ice during ice skating?
Answer: The weight of the body acts on a very small area of skate, in comparison to that of ice. Under this high pressure, ice melts to form water below 0°C. The water so formed makes the surface water skating more slippery. But as soon as the skater moves further, the water does not experience any pressure and re-freezes into ice leaving no track on ice surface.
Question 68: Why does evaporation causes cooling and why is water used in hot water bottles?
Answer: The specific latent heat of vaporization of water is 2260 J/g = 538 calories/g. Thus when water evaporates it absorbs large amount of latent heat and thus cooling is caused. The specific heat capacity of water is 4.2 J/kg°C = 1 cal/g°C which is quite high and this is the reason for using wafer in hot water bottles.
Question 69: What do you understand by the ‘latent heat of vaporization’ of a substance?
Answer: The latent heat of vaporization of a given liquid is the heat required to convert one kilogram of the liquid into vapour at its boiling point without any change of temperature. It is measured in joule per kilogram. The latent heat of vaporization of water is 2260 × 103 J/kg. It means that we need 2260 × 103 J of heat to convert 1 kg of water at its boiling point into steam.
Question 70: Explain the meaning of greenhouse effect.
Answer: The phenomenon of the trapping of infrared radiations of long wave length being radiated to space by greenhouse gases like carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O) to keep the environment warm at the planet’s surface and the lower atmosphere is called greenhouse effect.
Question 71: Name the greenhouse gases.
Answer: Carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), and water vapours.
Question 72: How does green house effect help in keeping the temperature of earth’s surface suitable for living human beings?
Answer: The greenhouse effect contribute in trapping the sun’s heat energy within the atmosphere to maintain Earth’s surface at an average temperature of about 59°F (15°C) which is suitable for living human beings.
Question 73: Give three reasons for the increase Of greenhouse gases.
Answer: (i) Combustion of fossil fuels, (transportation, power generation etc.)
(ii) Burning of forests and deforestations.
(iii) Increase of Population (human beings emit nearly 32 gig tonnes of carbon dioxide each year).
Question 74: What is meant by global warming?
Answer: Global warming means the change or increase in the average temperature of earth’s near surface air and oceans due to an increase in the amount of greenhouse gases in its atmosphere.
Question 75: State the effect-of enhancement of green house effect?
Answer: The increasing quantity of atmospheric carbon dioxide from the burning of fossil fuels, together with the release of other gases, is causing an increased greenhouse effect and this is leading to global warming.
Question 76: How will rise in sea level affect population in coastal countries?
Answer: Due to the rise in sea level the buildings and roads in the coastal areas will get flooded as a result population living in that area will suffer damage from hurricanes and tropical storms.
Question 77: What impact will global warming have on the health of the effected populations?
Answer: The increase in temperature due to global warming will result in many new diseases because certain bacteria are more effective and multiply much faster in warmer temperatures as compared to cold temperatures. It will also increase the rate of respiratory and Vector-borne disease infections like asthma and malaria respectively.
Question 78: State two impacts of global warming on the life on earth.
Answer: (i) It is responsible for the climate changes in different parts of the world and it has forced people to migrate from one place to the other.
(ii) It has affected blooming season of the different plants.
Question 79: State three ways to minimize global warming.
Answer: The three ways to minimize global warming are:
(i) Reduction in the use of fossil fuels and adoption of technology based on renewable sources of energy.
(ii) Reduction in deforestation and increase in afforestation.
(iii) Fostering international co-operation to reduce greenhouse gas emission.
Question 80: What impact will global warming have on the lakes and oceans?
Answer: The rising temperature will lower water quality in lakes and oceans through a fall in oxygen concentrations, release of phosphorus from sediments and increased thermal stability due to which many marine species will either die or they will become extinct.
Long Answers
Question 1: Explain briefly, how you would determine the specific heat capacity of a liquid?
Answer: In this case, take any solid of known specific heat and a liquid, whose specific heat capacity is to be determined. The solid chosen should be insoluble and non reactive with the liquid. The observations are to be noted as below:
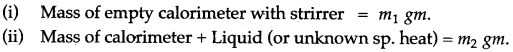
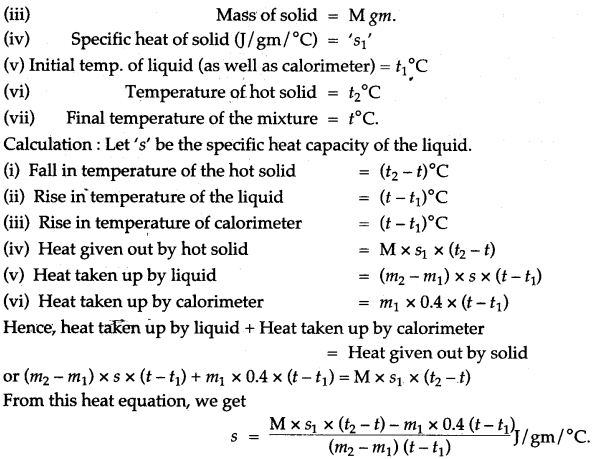
Question 2: Describe a method to determine the specific heat capacity of a solid (say, a piece of copper).
Answer: First we weigh the given piece of solid and note its mass m1. Then it is heated by suspending it inside a heater. Then a known mass (say m2) of water is taken in a thin glass beaker and its temperature θ1 is recorded with a thermometer. When the given piece of solid becomes heated, its temperature θ2 is noted and it is quickly dropped into the water contained in the beaker, such that no water splashes out. The contents of beaker are well stirred and the final highest temperature θ3 is noted.
Assuming that the heat capacity of beaker is negligible and there is not heat loss to the surroundings,
Heat lost by the solid = Heat gained by water Mass of solid × Specific heat capacity of solid × Fall in temperature of solid = Mass of water × Sp. capacity of water × Rise in temperature of water
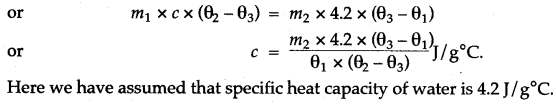
Question 3: State two advantages of the high specific latent heat capacity of steam, which is about 226 × 104 J/kg?
Answer: (i) The main advantage of the high specific latent heat capacity of steam is in room heating in cold countries. The steam generated in the boiler is passed through pipes in radiators fixed within the building.
(ii) In thermal power station, steam is used as a medium for converting the chemical energy of coal to electric energy. Every gram of steam supplies a great amount of heat energy of about 2260 joule.
Question 4: Discuss how high specific heat capacity of water helps in formation of land and sea breeze.
Answer: Specific heat capacity of land is 5 times less as compared to water. Thus, air above land becomes hot and light and rises up resulting in drop in pressure of land mass during day time. Thus, cool air from sea starts blowing towards and forming sea breeze.
During night, land as well as sea radiates heat energy. However, temperature of land falls faster than sea water, because of high specific heat capacity of sea water. So, at night the temperature of sea water is more than land. Warm air above the sea rises and cold air from land starts blowing towards sea resulting in land breeze.
Question 5: Will the value of specific heat’capacity and specific latent heat of a substance change if the scale is °F instead of °C?
The specific heat capacity will change if the scale is °F instead of °C. This is because it is the heat needed to raise the temperature of a unit of the substance by one degree. Since the size of the Fahrenheit degee is not the same as that of the celsium degree, a change of scale will affect the specific heat capacity.
The specific latent heat will not change with a change of scale. This is because this quantity is simply the heat needed to change the state of 1 kg of a substance at its melting/boiling point without any change in temperature. It is thus heat per unit mass and does not depends upon the scale of temperature used.
Question 6: State and explain the principle of ‘Calorimetry’.
Answer: The principle of calorimetry states:
When a hot body is brought in contact with a cold body, heat passes from the hot body to the cold body till both attain the same temperature.
Heat lost by hot body = Heat gained by cold body.
Let the mass of hot body be m1, its specific heat s1 and its temperature t1 °C and let the mass of cold body be m2, its specific heat s2 and its temperature t2 °C.

Question 7: State the main precautions to be taken in finding the latent heat of steam.
Answer: The main precautions are:
(i) Every possible care should be taken to see that steam is perfectly dry before passing into water.
(ii) The calorimeter should be properly insulated by keeping it within a wooden box provided with a sliding lid with two holes and lined inside with a layer of cotton wool, cotton pad, etc.
(iii) Steam should no longer be passed when temperature of water in the calorimeter rises approximately by 20°C.
(iv) While passing steam, care must be taken that condensed steam does not escape out.
Question 8: Derive an expression for the amount of heat given out or taken up, when its temperature falls or rises by t°C.
Answer: Let the mass of the body be ‘m’ and its specific heat capacity be ‘s’. It t°C is the rise in its temperature, then heat taken up by the body is calculated as below:
Since ‘s’ is the specific heat capacity of the body which means that the unit mass of the body for a rise of 1°C takes up ‘s’ amount of heat.
∴ Mass ‘m’ of the body for a rise of t°C will need heat
= m × s × t
= Mass of the body × Its specific heat × Rise in the temperature
= mst joule.
Figure Based Short Answers
Question 1: A substance is in the form of a solid at 0°C. The amount of heat added to this substance and the temperature of the substance are plotted on the following graph:
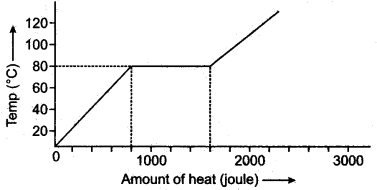
If the specific heat capacity of the solid substance is 500 J/kg °G, find from the graph, (i) the mass of the substance.
Solution: From graph heat supplied is 800 joule during change in temperature from 0 to 80°C.
Figure Based Long Answers
Question 1: A piece of ice is heated at a constant rate. The variation of temperature with heat input is shown in the graph below:
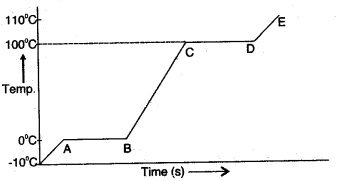
(i) What are represented by AB and CD?
(ii) What conclusion can you draw regarding the 110°c nature of ice from the above graph?
Answer: (i) AB part of the graph represents time in t seconds taken by ice at 0°C to convert into water at 0°C.
CD part of the graph represents time in seconds taken by water at 100°C to convert into steam at 100°C.
(ii) From the graph, we conclude that ice takes less time in heating from -10°C to 0°C. But in the process of melting, it takes comparatively longer time. It shows that ice has a high specific latent heat of fusion.
Question 2: What observation you will record and how will you determine the specific latent heat of fusion of ice?
Answer: A clean and dry copper calorimeter with a strirrer, is weighed when empty, again weighed when filled nearly 2/3 with water. Then initial temperature is noted. Some pieces of dry ice are transferred into the calorimeter. The calorimeter is placed inside the box and is continuously stirred till the whole of ice is melted. The final temperature is carefully noted. Then the mass of calorimeter with water and ice is again weighed.
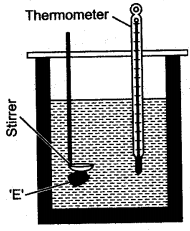
The observations recorded are:

If L j/gm be the specific latent heat of fusion of ice and sc be the specific heat capacity of copper, then:
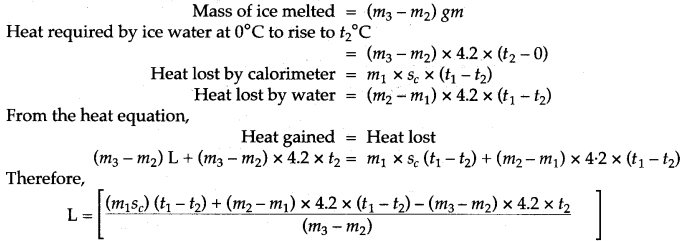
Short Numericals
Question 1: The temperature of a lead piece of mass 400 g rises from 20°C to 50°C when 1560 J of heat is supplied to it. Calculate.
(i) Heat capacity of lead piece, (ii) Specific heat capacity of lead.
Solution:

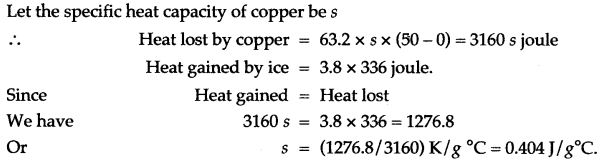
Question 2: 63.2 g of copper at 50°C can just melt 3.8g of ice. If the specific latent heat of ice is 336 J/g, find the specific heat capacity of copper.
Solution:
Question 3: Water falls from a height of 50 m. Calculate the rise in the temperature of water when it strikes the bottom.
(g = 10 ms-2; Specific heat capacity of water = 4200 J / kg°C)
Solution: If the whole of the energy due to fall of water changes into heat energy, and 0°C be the rice in the temperature of water, then
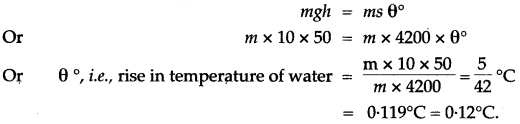
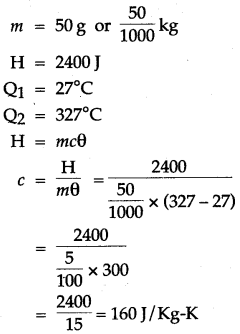
Question 4: 50 g of metal piece at 27°C requires 2400 J of heat energy so as to attain a temperature of 327°C. Calculate the specific heat capacity of the metal.
Solution:
Question 5: How much heat energy is released when 5 g of water at 20° C changes to ice at 0° C?
[Specific heat capacity of water = 4.2 J g-1 ° C-1 Specific latent heat of fusion of ice = 336 J g-1]
Solution:
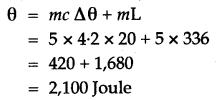
Question 6: A. hot solid of mass 60 g at 100°C is placed in 150 g of water at 20° C. The final steady temperature recorded is 25°C. Calculate the specific heat capacity of the solid. [Specific heat capacity of water = 4200 J kg-1 °C-1]
Solution:
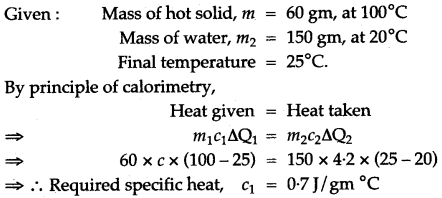
Question 7: Find the time taken by a 500 W heater to raise the temperature of 50 kg of material of specific heat capacity 960 J/kg°C from 18°C to 38°C. Assume that all the heat from the heater is given: to the material.
Solution:

Question 8: A piece of iron of mass 2.0 kg has a thermal capacity of 966 J/°C. What is its specific heat capacity in S.I. units?
Solution:
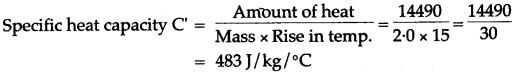
Question 9: A vessel of negligible heat*capacity contains 40g of ice in it at 0°C, 8g of steam at 100°C is passed into the ice to melt it. Find the final temperature of the contents of the vessel.
(Specific latent heat of vaporization of steam = 2268 J/g, specific latent heat of fusion of ice = 336 J/f and specific heat capacity of water = 4.2 J/g°C)
Solution: Assume, final temperature of contents = θ°C. Heat gained by ice at 0°C to water at 0°C + heat gained by water at 0°C to water at 0°C = Heat lost by steam at 100°C + Heat lost by water at 100°C to water at 0°C.
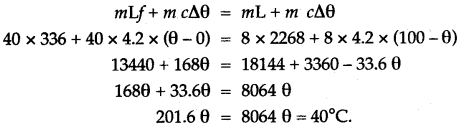
Question 10: Calculate the amount of heat released when 5.0 g of water at 20°C is changed into ice at 0°C.
(Specific heat capacity of water = 4.2 J/g°C
Specific latent heat of fusion of ice = 336 J/g).
Solution:
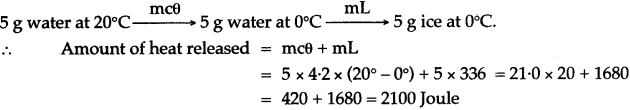
Question 11: Calculate the heat energy that will be released when 5.0 kg of steam at 100°C condenses to form water at 100°C. Express your answer in S.I. unit. (Specific latent heat of vaporization of steam is 2268 kj/kg.)
Solution: Heat released by 5 kg of steam at 100°C to convert into water at 100°C. = mL
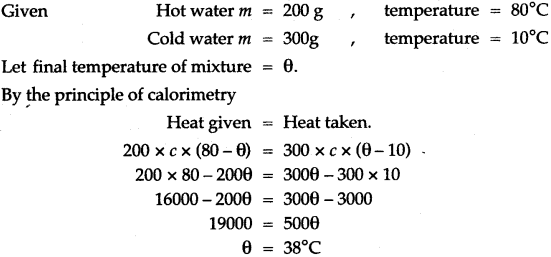
= 5 × 2268 KJ = 11440 kj.
Question 12: 200 g of hot water at 80°C is added to 300 g of cold water at 10 °C. Calculate the final temperature of the mixture of water. Consider the heat taken by the container to be negligible. [specific heat capacity of water is 4200 J kg-1 °C-1]
Solution:
Question 13: An electric immersion heater is rated 1250 W. Calculate the time in which it will heat 20 kg of water at 5°C to 65°C.
Solution:

Question 14: A piece of iron of mass 2.0 kg has a thermal capacity of 966 J/°C. How much heat is needed to warm it by 15°C?
Solution: m = 2.0kg, Thermal capacity = 966 J/°C
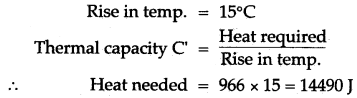
Question 15: Some hot water was added to three times its mass of cold water at 10°C and the resulting temperature was found to be 20°C. What was the temperature of the hot water?
Solution: Let the temperature of hot water be t°C and mass of hot water = m gm
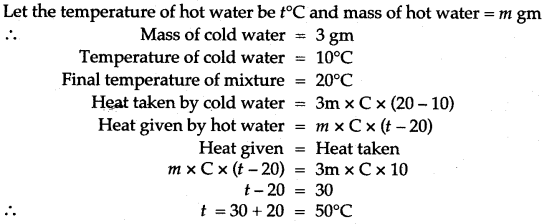
Time required is 1hr, 7 min, 12 sec.
Long Numericals
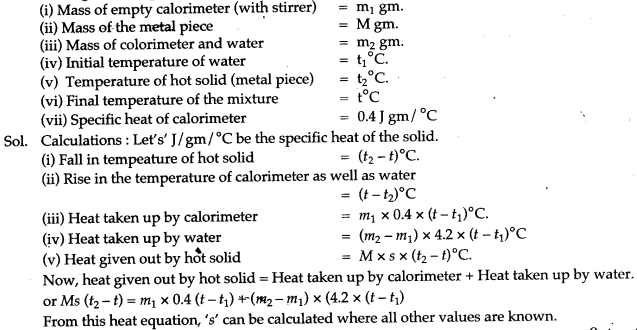
Question 1: Derive an expression for finding out the specific heat capacity of a body (solid) from the readings of an experiment given below:
Solution:
Question 2: A body ‘A’ of mass m1 of a substance of a specific heat capacity c1 at a temperature θ1 is mixed with a body ‘B’ of mass m2 of other substance of specific heat capacity c2 at a lower temperature θ2. Deduce the expression for the temperature of the mixture. State the assumption made, if any.
Solution:
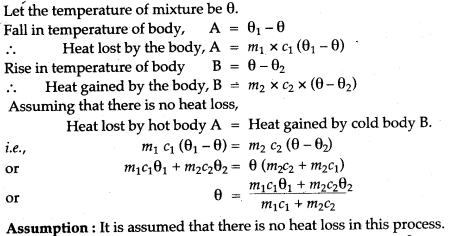
Question 3: A copper calorimeter of mass 50g contains 100g of water at 20°C. A metallic piece of mass 250 g is heated to 100°C and is then dropped into the calorimeter. The contents of the calorimeter are well stirred and its final highest temperature is recorded to be 28 °C. If the specific heat capacity of water is 4.2 J/g°C and of copper is 0.4 J/g°C, find: (i) the heat gained by water, (ii) the heat gained by calorimeter, (iii) total heat supplied by the metal piece, and (iv) the specific heat capacity of metal.
Answer:
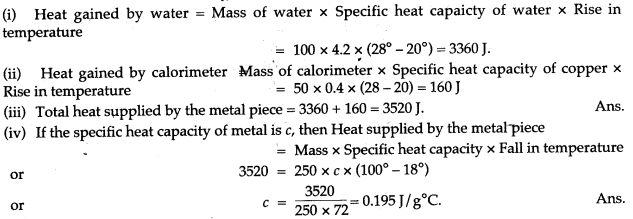
Question 4: A mass of 40g of brass of specific heat capacity 0.85 Jg-1 K-1 is heated in an oven and then quickly transferred into 240g of water at 30°C in a calorimeter of mass 60g and specific heat capacity 0.4 Jg-1 K-1. If the final temperature is 50°C. What was the temperature of the oven?
Solution:
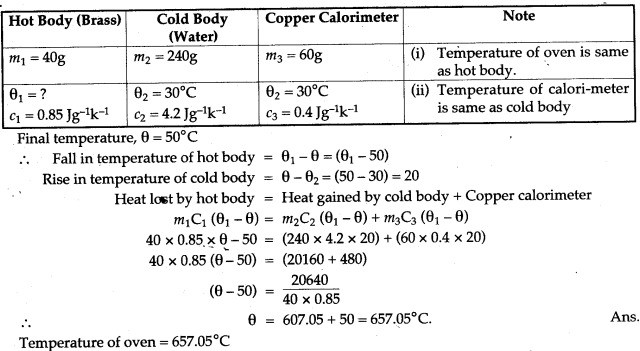
Question 5: A Bunsen burner raises the temperature of 500g of water from 10°C to 100°C in 5 minutes. What heat is supplied per second?
Solution:
Question 6: 40g of ice at 0°C is used to bring down the temperature of a certain mass of water at 60°C to 10°C. Find the mass of water used.
[Specific heat capacity of water = 4200 J kg-1 °C-1]
[Specific latent heat of fusion of ice = 336 × 103 J kg-1]
Solution: Let mass of water used = m gm.

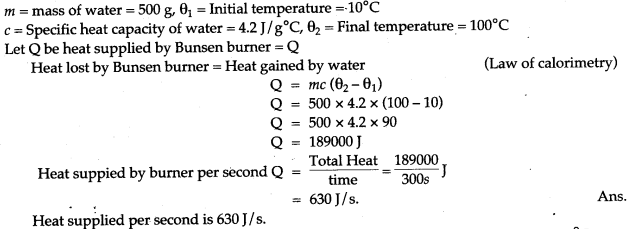
Question 7: 250 g of water at 30 °C is present in a copper vessel of mass 50 g. Calculate the mass of ice required to bring down the temperature of the vessel and its contents to 5 °C.
Specific latent heat of fusion of ice = 336 × 103 J kg-1
Specific heat capacity of copper vessel = 400 J kg-1 °C-1
Specific heat capacity of water = 4200 J kg-1 °C-1.
Solution:
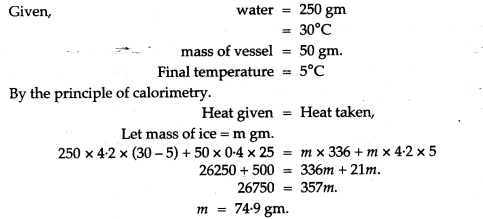
Question 8: The temperature of 600 g of cold water rise by 15°C when 300 g of hot water at 50°C was added to it. What was the initial temperature?
Solution:

Question 9: 20 g of ice at 0°C is added to 200g of water at 20°C. Calculate the drop in temperature ignoring the heat capacity of the container. (Specific latent heat of ice = 80 cal/g)
Solution:

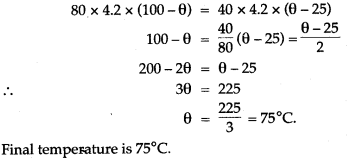
Question 10: Find the final temperature when a mass of 80g of water at 100°C is mixed with a mass of 40g of water at 25°C.
Solution:

Question 11: A heater supplies heat at the rate of 800 J/s. Find the time required to convert 50g of ice at -20°C into superheated steam at 140°C. (Given: Specific heat of ice = 2.1 J/g, latent heat of ice = 340 J/gm, latent heat of steam = 2240 J/gm, specific heat of steam = 2.1 J/gm and specific heat of water = 4.2 J/gm.)
Solution:
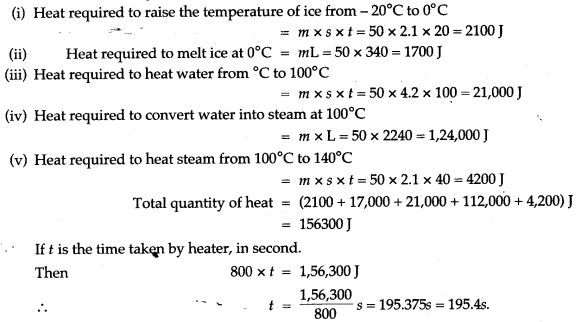
Question 12: (i) A molten metal weighing 150 g is kept at its melting point 800°C. When it is allowed to solidify at the same temperature, it gives out 75000 J of heat. What is the sp. latent heat of the metal?
(ii) If its sp. heat capacity is 200 J/kg-K, how much additional heat will it give out in cooling to-50°C?
Solution:
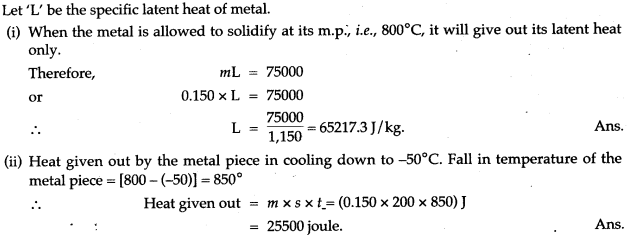
Question 13: Heat energy is supplied at a constant rate to 100 g of ice at 0°C. The ice is converted into water at 0°C in 2 minutes. How much time will be required to raise the temperature of water from 0°C to 20°C? [Given: sp. heat capacity of water 4.2 g-1 °C-1, sp. latent heat of ice = 336 J g-1]
Solution:
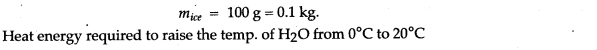
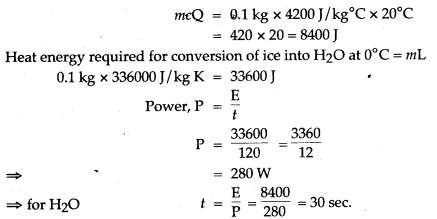
Question 14: 50 g of ice at 0°C is added to 300 g of a liquid at 30°C. What will be the final temperature of the mixture when all the ice has melted? The specific heat capacity of the liquid is 2.65 Jg-1 °C-1 while that of water is 4.2 J g-1 °C-1. Specific latent heat of fusion of ice = 336 Jg-1.
Solution:

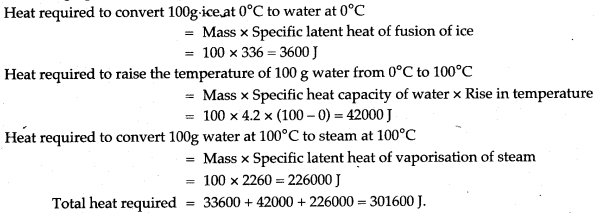
Question 15: Calculate the total amount of heat required to convert 100g ice at 0°C to steam at 100°C. (Specific latent heat of fusion of ice = 336 J/g, specific latent heat of vaporisation of steam = 2260 J/g, specific heat capacity of water = 4.2 J/g°C).
Solution:
Question 16: Steam at 100°C is passed over 1000 g of ice at 0°C. After some time, 600 g of ice at 0°C is left and 450 g of water at 0°C is formed. Calculate the specific latent heat of vaporsation of steam (Given: specific heat capacity of water = 4200 J/kg°C, specific latent heat of fusion of ice = 336,000 J/kg.)
Solution:
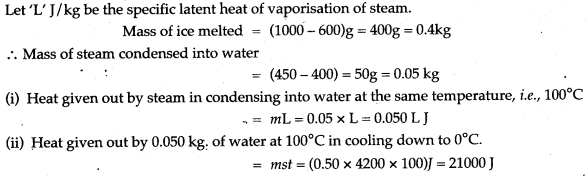

Question 17: If there is no Heat loss to the surroundings, the heat released by the condensation of m1 g of steam at 100°C into water at 100°C can be used to convert m2 g of ice at 0°C into water at 0°C.

Solution:

Question 18: 1 kg of water is contained in a 1.25 kW kettle. Assuming specific heat capacity of water = 4.2 J/g °C and specific latent heat of vaporisation = 2260 J/g, calculate: (i) the time taken for the temperature of water to rise from 25°C to its boiling point, (ii) the mass of water which evaporates per minute from the boiling water.
Solution:

For More Resources