Why is energy released when a bond is formed?
Energy change during formation and breaking of bonds
- During a chemical reaction, the bonds between the atoms in the reactans need to be broken first before new bonds between the atoms in the products can be formed.
- Chemical reactions involve bond breaking and bond formation:
(a) Bond breaking requires energy. Thus, bond breaking is an endothermic change.
(b) Bond formation releases energy. Thus, bond formation is an exothermic process. - The quantity of heat energy absorbed or released during the breaking and formation of bonds in a chemical reaction depends on the strength of the bond.
(a) More energy is required to break a strong bond compared to a weak bond.
(b) More energy is released when a strong bond is formed compared to a weak bond. - Table shows the average bond energies of some common covalent bonds.
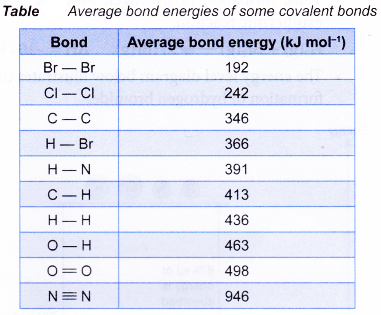
- The heat absorbed or given out in a reaction comes from the bonds being broken or made in the reaction.
ΔH = Eb– Ef
where,
Eb is the total energy absorbed during the breaking of bonds, and
Ef is the total energy given out during the formation of bonds.
(a) If bond formation releases more energy than is required in the bond breaking (Ef > Eb), ΔH = Eb – Ef = negative. The reaction is exothermic.
(b) If bond breaking requires more energy than the energy that is released during bond formation (Ef > Eb), ΔH = Eb – Ef = positive. The reaction is endothermic.
People also ask
- How can energy be changed in a chemical reaction?
- How does the energy level diagram show this reaction is exothermic?
- What is enthalpy of reaction?
- What is heat of precipitation?
- What is heat of displacement?
- What is the enthalpy of neutralization?
- What is the heat of combustion?
- Applications of exothermic and endothermic reactions in everyday life
- What is meant by the calorific value of a fuel?
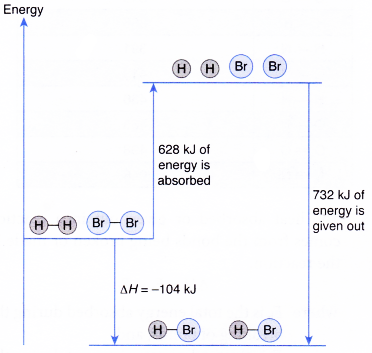
Formation of hydrogen bromide, HBr :
H2(g) + Br2(g) → 2HBr(g)
H – H + Br – Br → 2 H – Br
- Total energy absorbed to break the bonds in the reactants,
Eb = energy to break 1 mole of H – H bonds + energy to break 1 mole of Br – Br bonds
= 436 kJ + 192 kj = 628 kJ - Total energy given out to form the bonds in the products,
Ef = energy to form 2 moles of H – Br
= 2 x 366 kJ = 732 kJ - Overall heat change in the reaction,
ΔH = Eb – Ef
= 628 kJ – 732 kJ = -104 kJ - Thus, formation of hydrogen bromide is an exothermic reaction.
H2(g) + Br2(g) → 2HBr(g) ΔH = -104 kJ - The energy level diagram below illustrates the formation of hydrogen bromide.
Decomposition of ammonia:
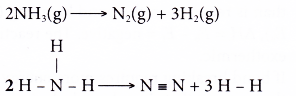
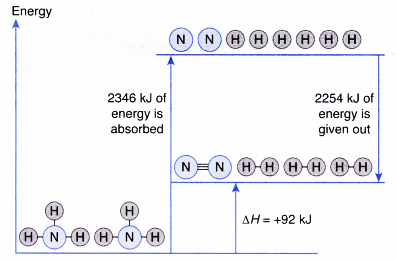
- Total energy absorbed to break the bonds in the reactants,
Eb = energy to break 6 moles of H – N bonds
= 6 x 391 kJ = 2346 kJ - Total energy given out to fo.rm the bonds in the products, Ef

- Overall heat change in the reaction,
ΔH = Eb-Ef
= 2346 kJ – 2254 kJ = +92 kJ
The decomposition of ammonia to form nitrogen and hydrogen is an endothermic reaction.
2NH3(g) → N2(g) + 3H2(g) ΔH = +92 kJ - The energy level diagram below illustrates the decomposition of ammonia.
Example: The following is the chemical equation of the formation of hydrogen chloride.
H2(g) + Cl2(g) → 2HCl(g)
Calculate the overall heat change in the reaction.
Given that the bond energy of H – H = 436 kJ mol-1, Cl – Cl = 242 kJ mol-1, H – Cl = 432 kJ mol-1.
Solution:
