What is radio active decay?
Radioactive Decay:
- A stable nucleus of an element has the correct balance of protons and neutrons.
- Isotopes of an element which have too few or too many neutrons are usually unstable.
- Carbon-12 is stable but carbon-14 which has 2 extra neutrons is unstable. Nitrogen-14 is stable but its isotope, nitrogen-13 which has 1 neutron less is unstable.
- An unstable nucleus emits radiation in the form of an alpha particle, a beta particle or gamma rays to become a more stable nucleus.
- Radioactive decay is the process in which an unstable nucleus changes into a more stable nucleus by emitting radiation.
- In radioactive decay, the parent nuclide. emits radiation and changes into a daughter nuclide.
- Radioactive decay is named after the type of radiation emitted.
People also ask
- Radioactivity: Types of Radioactive Emissions
- How do you detect radioactivity?
- What are the Isotopes, Isobars and Isotones of an Element
- What is the Radioactive Isotope?
- What is the half life of a radioactive element?
- Importance of Proper Management of Radioactive Substances
- What is nucleus of an atom?
- What is Nuclear Energy?
- What is nuclear fission and how does it occur?
- How is energy released in a nuclear fusion reaction?
- What happens in a nuclear chain reaction?
- How does a nuclear power plant works?
What are the different types of radioactive decay?
There are three types of radioactive decay:
(a) Alpha decay
(b) Beta decay
(c) Gamma decay
Alpha Decay
- In alpha decay, the unstable parent nuclide emits an alpha particle.
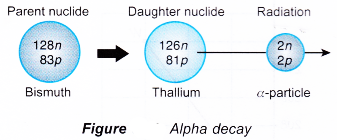
- Heavier unstable nuclei are more likely to undergo alpha decay. A bismuth-211 (83Bi211) nucleus is unstable and emits an alpha particle. Lawrencium-257 (103Lr257) also emits alpha particles.
- Figure shows a diagrammatic representation of the decay of bismuth-211.
- The equation for the decay of bismuth-211 is as follows:
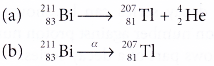
- The daughter nuclide has 2 protons less and 2 neutrons less than the parent nuclide. This means that in alpha decay, the proton number is reduced by 2 while the nucleon number is reduced by 4.
- The general equation for alpha decay can be written as:

Beta Decay
- In beta decay, the parent nuclide emits a beta particle.
- Usually heavier unstable nuclei with an excess of neutrons will undergo beta decay.
(a) Carbon-12 is stable but a carbon-14 (6C14) nucleus is unstable and emits a beta particle.
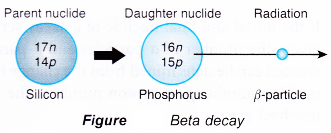
(b) Silicon-28 is stable but silicon-31 (14Si31) with three extra neutrons will emit a beta particle. - During beta decay, one of the neutrons changes into a proton and an electron, as shown by the equation below.
 The proton remains in the nucleus while the electron is emitted as a beta particle.
The proton remains in the nucleus while the electron is emitted as a beta particle. - This means that in beta decay, the nucleus loses a neutron but gains a proton.
- Figure shows a diagrammatic representation of the decay of silicon-31.
- The equation for the decay of silicon-31 is as follows:
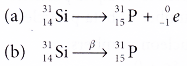
- The proton number increases by one because of the new proton formed but the nucleon number is unchanged because of the loss of one neutron.
- The general equation for beta decay can be written as:

- Other examples of nuclides which undergo beta decay are phosphorus-32, strontium-90, iodine-131 and actinium-228.
Gamma Decay
- In gamma decay, a nucleus in an excited state (higher energy state) emits a γ-ray photon to change to a lower energy
- state.
There is no change in the proton number and nucleon number. - A cobalt-60 nucleus in the excited state emits a y-ray photon. The equation for the decay is:
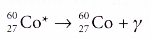 The (*) denotes the nucleus is in an excited state.
The (*) denotes the nucleus is in an excited state. - The general equation for gamma decay can be written as:
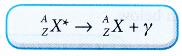
- The emission of γ-rays often accompany the emission of α-particles and β-particles. The following are some examples of these decays.
Radioactive Decay Series
![]()
- Sometimes the daughter nuclide of a radioactive decay is still unstable. It will eventually decay into another nuclide which is also unstable.
- This process continues as a radioactive decay series until a stable nuclide is reached. Each decay will emit either an a-particle or a β-particle and may be accompanied by γ-rays.
- Polonium-218 goes through a series of seven decays to become a stable lead-206 atom, as shown in Figure. Three a-particles and four β-particles are emitted in the process.
- If the initial and final nuclide of a decay series is given, the number of a-particles and β-particles emitted can be determined from the change in the nucleon number and proton number. The steps involved are:
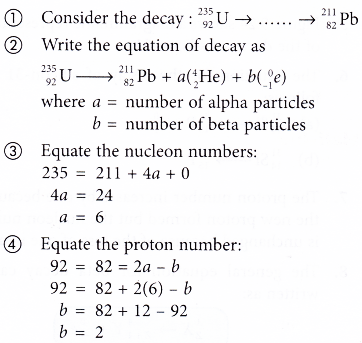
Therefore, 6 alpha particles and 2 beta particles are emitted. - A radioactive decay series can be shown on a graph of nucleon number against proton number. Figure shows part of a decay series.
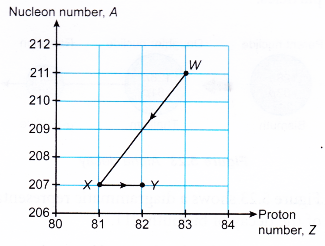
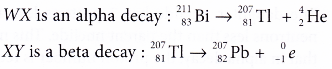
Radioactive Decay Example Problems with Solutions
- State the type of decay and balance the equation.
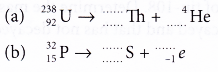
Solution:
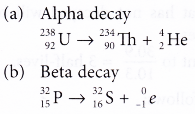
- Nuclide 83Bi206 undergoes a series of decays to become 82Pb206. Determine the number of alpha particles and beta particles emitted in this decay series.
Solution:
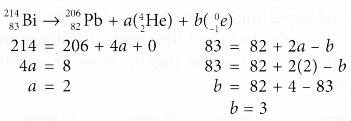
Therefore, 2 alpha particles and 3 beta perticles are emitted.